-
Name
11-KETOTESTOSTERONE
- EINECS
- CAS No. 564-35-2
- Article Data14
- CAS DataBase
- Density 1.191 g/cm3
- Solubility
- Melting Point 183-184 °C(Solv: acetone (67-64-1); hexane (110-54-3))
- Formula C19H26O3
- Boiling Point 476.791 °C at 760 mmHg
- Molecular Weight 302.414
- Flash Point 256.265 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Androst-4-ene-3,11-dione,17β-hydroxy- (8CI);11-Ketotestosterone;11-Oxotestosterone;11-keto-Testosterone;17β-Hydroxyandrost-4-ene-3,11-dione;4-Androsten-17β-ol-3,11-dione;
- PSA 54.37000
- LogP 3.05820
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In pyridine; methanol | 67% |
| With tris-buffer; 1,4-dihydronicotinamide adenine dinucleotide; 17β-hydroxysteroid dehydrogenase In ethylene glycol for 1h; Ambient temperature; | 15% |
| With sodium tetrahydroborate |
-
-
81176-76-3
(8S,9S,10R,13S,14S,17S)-10,13-Dimethyl-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthrene-3,11,17-triol

-
A
-
382-45-6
adrenosterone

-
B
-
564-35-2
11-ketotestosterone

| Conditions | Yield |
|---|---|
| With 4-(N,N-dimethylamino)pyridinium chlorochromate In dichloromethane for 4h; | A 3% B 49% |

| Conditions | Yield |
|---|---|
| With bromine dann mit Semicarbazid und anschliessend mit Brenztraubensaeure; |

-
-
564-35-2
11-ketotestosterone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 91 percent / pyridinium dichromate / CH2Cl2 / 44 h / Ambient temperature 2: 15 percent / tris-buffer (pH 7.4), 17β-hydroxysteroid dehydrogenase, β-NADH / ethane-1,2-diol / 1 h / Ambient temperature View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 69 percent / CrO3, H2SO4 / acetone 2: 67 percent / NaBH4 / methanol; pyridine View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: CrO3 View Scheme |
-
-
35271-42-2
11β-hydroxy-17β-propionyloxy-androst-4-en-3-one

-
-
564-35-2
11-ketotestosterone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: CrO3 View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: ethylene glycol; benzene; toluene-4-sulfonic acid 2: platinum / Hydrogenation.und anschliessenden Hydrolyse 3: bromine / dann mit Semicarbazid und anschliessend mit Brenztraubensaeure View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: N-bromo-acetamide 2: ethylene glycol; benzene; toluene-4-sulfonic acid 3: platinum / Hydrogenation.und anschliessenden Hydrolyse 4: bromine / dann mit Semicarbazid und anschliessend mit Brenztraubensaeure View Scheme |
-
-
739-27-5, 1231-82-9, 7090-90-6, 28336-28-9, 40272-95-5, 94991-82-9, 109838-19-9
3α-hydroxy-5β-androstane-11,17-dione

-
-
564-35-2
11-ketotestosterone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: N-bromo-acetamide 2: ethylene glycol; benzene; toluene-4-sulfonic acid 3: platinum / Hydrogenation.und anschliessenden Hydrolyse 4: bromine / dann mit Semicarbazid und anschliessend mit Brenztraubensaeure View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: ueber mehrere Stufen 2: mit Hilfe von Saccharomyces cerevisiae View Scheme | |
| Multi-step reaction with 5 steps 1: NaBH4 / anschliessend mit Blei(IV)-acetat bzw. NaBiO3 2: N-bromo-acetamide 3: ethylene glycol; benzene; toluene-4-sulfonic acid 4: platinum / Hydrogenation.und anschliessenden Hydrolyse 5: bromine / dann mit Semicarbazid und anschliessend mit Brenztraubensaeure View Scheme |
-
-
113278-74-3
3,3-ethanediyldioxy-5β-androstane-11,17-dione

-
-
564-35-2
11-ketotestosterone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: platinum / Hydrogenation.und anschliessenden Hydrolyse 2: bromine / dann mit Semicarbazid und anschliessend mit Brenztraubensaeure View Scheme |

| Conditions | Yield |
|---|---|
| With pyridine; dmap at 20℃; for 24h; | 86% |
-
-
564-35-2
11-ketotestosterone

-
-
108-24-7
acetic anhydride

-
-
16375-27-2
17β-Acetoxy-5α-androstan-3.11-dion

| Conditions | Yield |
|---|---|
| (i) H2, Pd-C, EtOH, (ii) /BRN= 385737/, Py; Multistep reaction; |
-
-
564-35-2
11-ketotestosterone

-
-
108-24-7
acetic anhydride

-
A
-
16375-27-2
17β-Acetoxy-5α-androstan-3.11-dion

| Conditions | Yield |
|---|---|
| (i) H2, Pd-C, EtOH, (ii) /BRN= 385737/, Py; Multistep reaction; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: (i) H2, Pd-C, EtOH, (ii) /BRN= 385737/, Py 2: aq. KOH / methanol View Scheme | |
| With aldo-keto reductase family member 1 type D1 Kinetics; Enzymatic reaction; |
-
-
564-35-2
11-ketotestosterone

-
-
32694-37-4
17β-dihydroxy-5α-androstan-3,11-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: (i) H2, Pd-C, EtOH, (ii) /BRN= 385737/, Py 2: aq. KOH / methanol View Scheme | |
| Multi-step reaction with 2 steps 1: (i) H2, Pd-C, EtOH, (ii) /BRN= 385737/, Py 2: aq. KOH / methanol View Scheme | |
| With steroid 5α-reductase type 2 Kinetics; Reagent/catalyst; Enzymatic reaction; |
-
-
564-35-2
11-ketotestosterone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: (i) H2, Pd-C, EtOH, (ii) /BRN= 385737/, Py 2: Br2, HBr, NaOAc / acetic acid 3: Li2CO3, LiBr / dimethylformamide View Scheme | |
| Multi-step reaction with 3 steps 1: (i) H2, Pd-C, EtOH, (ii) /BRN= 385737/, Py 2: Br2, HBr, NaOAc / acetic acid 3: Li2CO3, LiBr / dimethylformamide View Scheme |
-
-
564-35-2
11-ketotestosterone

-
-
98878-62-7
2α-Brom-17β-acetoxy-5α-androstan-3.11-dion

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: (i) H2, Pd-C, EtOH, (ii) /BRN= 385737/, Py 2: Br2, HBr, NaOAc / acetic acid View Scheme | |
| Multi-step reaction with 2 steps 1: (i) H2, Pd-C, EtOH, (ii) /BRN= 385737/, Py 2: Br2, HBr, NaOAc / acetic acid View Scheme |
-
-
564-35-2
11-ketotestosterone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: dmap; pyridine / 24 h / 20 °C 2: pyridine; hydroxylamine hydrochloride / ethanol / 24 h / 20 °C View Scheme |
-
-
564-35-2
11-ketotestosterone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: dmap; pyridine / 24 h / 20 °C 2: pyridine; hydroxylamine hydrochloride / ethanol / 24 h / 20 °C 3: thionyl chloride / 1,4-dioxane / 24 h / 0 - 20 °C View Scheme |
-
-
564-35-2
11-ketotestosterone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: dmap; pyridine / 24 h / 20 °C 2: pyridine; hydroxylamine hydrochloride / ethanol / 24 h / 20 °C 3: thionyl chloride / 1,4-dioxane / 24 h / 0 - 20 °C 4: methanol; lithium hydroxide / 1 h / 20 °C View Scheme |
-
-
564-35-2
11-ketotestosterone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: dmap; pyridine / 24 h / 20 °C 2: pyridine; hydroxylamine hydrochloride / ethanol / 24 h / 20 °C 3: thionyl chloride / 1,4-dioxane / 24 h / 0 - 20 °C 4: methanol; lithium hydroxide / 1 h / 20 °C 5: dicyclohexyl-carbodiimide; dmap / dichloromethane / 24 h / 20 °C View Scheme |
-
-
564-35-2
11-ketotestosterone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: dmap; pyridine / 24 h / 20 °C 2: pyridine; hydroxylamine hydrochloride / ethanol / 24 h / 20 °C 3: thionyl chloride / 1,4-dioxane / 24 h / 0 - 20 °C 4: pyridine; hydroxylamine hydrochloride / ethanol / 168 h / 140 °C / Sealed tube View Scheme |
-
-
564-35-2
11-ketotestosterone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: dmap; pyridine / 24 h / 20 °C 2: pyridine; hydroxylamine hydrochloride / ethanol / 24 h / 20 °C 3: thionyl chloride / 1,4-dioxane / 24 h / 0 - 20 °C 4: pyridine; hydroxylamine hydrochloride / ethanol / 168 h / 140 °C / Sealed tube 5: thionyl chloride / 1,4-dioxane / 24 h / 0 - 20 °C View Scheme |
-
-
564-35-2
11-ketotestosterone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: dmap; pyridine / 24 h / 20 °C 2: pyridine; hydroxylamine hydrochloride / ethanol / 24 h / 20 °C 3: thionyl chloride / 1,4-dioxane / 24 h / 0 - 20 °C 4: pyridine; hydroxylamine hydrochloride / ethanol / 168 h / 140 °C / Sealed tube 5: thionyl chloride / 1,4-dioxane / 24 h / 0 - 20 °C 6: methanol; lithium hydroxide / 1 h / 20 °C View Scheme |
-
-
564-35-2
11-ketotestosterone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: dmap; pyridine / 24 h / 20 °C 2: pyridine; hydroxylamine hydrochloride / ethanol / 24 h / 20 °C 3: thionyl chloride / 1,4-dioxane / 24 h / 0 - 20 °C 4: pyridine; hydroxylamine hydrochloride / ethanol / 168 h / 140 °C / Sealed tube 5: thionyl chloride / 1,4-dioxane / 24 h / 0 - 20 °C 6: methanol; lithium hydroxide / 1 h / 20 °C 7: dicyclohexyl-carbodiimide; dmap / dichloromethane / 24 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: aldo-keto reductase family member 1 type D1 / Enzymatic reaction 2: 3α-hydroxysteroid dehydrogenase type 3 / Enzymatic reaction 3: 17β-hydroxysteroid dehydrogenase type 2 / Enzymatic reaction View Scheme |
11-Ketotestosterone Specification
The 11-Ketotestosterone, with the CAS registry number 50-67-9, is also known as 4-Androsten-17β-ol-3,11-dione. It belongs to the product categories of Intermediates & Fine Chemicals; Metabolites & Impurities; Pharmaceuticals; Steroids. This chemical's molecular formula is C19H26O3 and molecular weight is 302.41. What's more, its systematic name is (17β)-17-Hydroxyandrost-4-ene-3,11-dione. This chemical is used as a metabolite of Adrenosterone.
Physical properties of 11-Ketotestosterone are: (1)ACD/LogP: 1.296; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.30; (4)ACD/LogD (pH 7.4): 1.30; (5)ACD/BCF (pH 5.5): 5.69; (6)ACD/BCF (pH 7.4): 5.69; (7)ACD/KOC (pH 5.5): 120.86; (8)ACD/KOC (pH 7.4): 120.86; (9)#H bond acceptors: 3; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 54.37 Å2; (13)Index of Refraction: 1.569; (14)Molar Refractivity: 83.229 cm3; (15)Molar Volume: 253.886 cm3; (16)Polarizability: 32.995×10-24cm3; (17)Surface Tension: 48.2 dyne/cm; (18)Density: 1.191 g/cm3; (19)Flash Point: 256.265 °C; (20)Enthalpy of Vaporization: 85.361 kJ/mol; (21)Boiling Point: 476.791 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25°C.
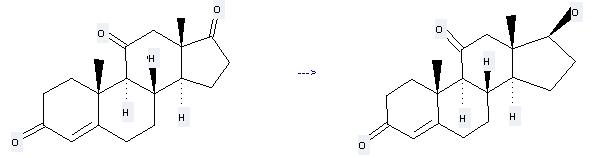
Preparation of 11-Ketotestosterone: this chemical can be prepared by androst-4-ene-3,11,17-trione. This reaction will need reagent NaBH4 and solvents methanol, pyridine. The yield is about 67%.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C2C[C@]4([C@H]([C@@H]3CC\C1=C\C(=O)CC[C@]1(C)[C@@H]23)CC[C@@H]4O)C
(2)Std. InChI: InChI=1S/C19H26O3/c1-18-8-7-12(20)9-11(18)3-4-13-14-5-6-16(22)19(14,2)10-15(21)17(13)18/h9,13-14,16-17,22H,3-8,10H2,1-2H3/t13-,14-,16-,17+,18-,19-/m0/s1
(3)Std. InChIKey: WTPMRQZHJLJSBO-XQALERBDSA-N
Related Products
- 11-Ketotestosterone
- 56441-55-5
- 56441-69-1
- 56442-17-2
- 56442-19-4
- 56444-80-5
- 564469-85-8
- 56447-54-2
- 564483-18-7
- 56449-18-4
- 56-45-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View