-
Name
5-METHYL-1H-PYRROLE-2-CARBALDEHYDE
- EINECS
- CAS No. 1192-79-6
- Article Data30
- CAS DataBase
- Density 1.14 g/cm3
- Solubility
- Melting Point 68 °C
- Formula C6H7NO
- Boiling Point 226.1 °C at 760 mmHg
- Molecular Weight 109.128
- Flash Point 95.8 °C
- Transport Information
- Appearance
- Safety 26
- Risk Codes 36
-
Molecular Structure
- Hazard Symbols
- Synonyms 5-Methyl-2-formylpyrrole;
- PSA 32.86000
- LogP 1.13560
Synthetic route
-
-
636-41-9
2-methyl-1H-pyrrole

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
1192-79-6
formyl-2 methyl-5 pyrrole

| Conditions | Yield |
|---|---|
| With trichlorophosphate | 70% |
| With trichlorophosphate In 1,2-dichloro-ethane 1) 0 deg C, 1 h, 2) up to r.t., 3) reflux, 30 min; | 64.5% |
| Stage #1: N,N-dimethyl-formamide With trichlorophosphate In 1,2-dichloro-ethane at 0 - 20℃; for 0.25h; Stage #2: 2-methyl-1H-pyrrole In 1,2-dichloro-ethane at 0 - 80℃; for 0.5h; Stage #3: With water; sodium acetate In 1,2-dichloro-ethane at 80℃; for 0.333333h; | 56% |
| With trichlorophosphate 1.)from 0 deg C to 20 deg C, 15 min; 2.)ethylene chloride, ethylene dichloride, 15 min, reflux; Yield given. Multistep reaction; | |
| With trichlorophosphate In 1,2-dichloro-ethane |
-
-
124647-46-7
N-(hydroxy-2' ethyl) amino-3 butene-2 oate d'ethyle

-
A
-
1192-79-6
formyl-2 methyl-5 pyrrole

-
B
-
17619-39-5
2-methyl-1H-pyrrole-3-carbaldehyde

-
C
-
124647-59-2
formyl-1 methyl-2 pyrrole

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 400℃; sealed tube; | A 30% B 23% C 9% |
| In tetrahydrofuran at 400℃; sealed tube; | A n/a B 23% C n/a |
| In tetrahydrofuran at 400℃; sealed tube; | A n/a B n/a C 9% |


| Conditions | Yield |
|---|---|
| Stage #1: N,N-dimethyl-formamide With trichlorophosphate for 0.333333h; Stage #2: 2-methyl-1H-pyrrole In 1,2-dichloro-ethane for 0.25h; Heating / reflux; Stage #3: With sodium acetate In 1,2-dichloro-ethane at 80℃; for 0.333333h; | 18% |
-
-
636-41-9
2-methyl-1H-pyrrole

-
-
60-29-7
diethyl ether

-
-
925-90-6
ethylmagnesium bromide

-
-
109-94-4
formic acid ethyl ester

-
-
1192-79-6
formyl-2 methyl-5 pyrrole

| Conditions | Yield |
|---|---|


| Conditions | Yield |
|---|---|
| With diethyl ether; ethylmagnesium bromide |
-
-
4205-23-6
D-gulose

-
-
56-40-6
glycine

-
A
-
1192-79-6
formyl-2 methyl-5 pyrrole

-
B
-
1121-78-4
5-Hydroxy-2-methylpyridine

-
C
-
1121-25-1
2-methyl-3-pyridinol

-
D
-
1072-83-9
2-Acetylpyrrole

| Conditions | Yield |
|---|---|
| In water at 100℃; for 24h; Product distribution; pH 2.0; other pH; times; |

-
-
4134-97-8, 30382-30-0, 50455-94-2, 82399-12-0, 4084-27-9
3-deoxyglucosone

-
-
56-40-6
glycine

-
A
-
1192-79-6
formyl-2 methyl-5 pyrrole

-
B
-
1121-78-4
5-Hydroxy-2-methylpyridine

-
C
-
1121-25-1
2-methyl-3-pyridinol

| Conditions | Yield |
|---|---|
| In water at 100℃; for 24h; Product distribution; pH 3.0; |

-
-
4429-05-4
N-(1-deoxy-D-fructos-1-yl)-L-glycine

-
-
56-40-6
glycine

-
A
-
1192-79-6
formyl-2 methyl-5 pyrrole

-
B
-
1121-78-4
5-Hydroxy-2-methylpyridine

-
C
-
1121-25-1
2-methyl-3-pyridinol

-
D
-
1072-83-9
2-Acetylpyrrole

| Conditions | Yield |
|---|---|
| In water at 100℃; for 24h; Product distribution; pH 3.0; |

-
-
64435-30-9
N5,N5,N10,N10-tetramethyl-5,10-dihydrodipyrrolo[1,2-a:1',2'-d]pyrazine-5,10-diamine

-
-
77-78-1
dimethyl sulfate

-
-
1192-79-6
formyl-2 methyl-5 pyrrole

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction; |
-
-
64435-30-9
N5,N5,N10,N10-tetramethyl-5,10-dihydrodipyrrolo[1,2-a:1',2'-d]pyrazine-5,10-diamine

-
-
74-88-4
methyl iodide

-
-
1192-79-6
formyl-2 methyl-5 pyrrole

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With sodium acetate 1) from -78 deg C to RT; 2) water, 15 h, reflux; Yield given. Multistep reaction; |
-
-
2280-44-6
D-Glucose

-
A
-
98-00-0
(2-furyl)methyl alcohol

-
B
-
1192-79-6
formyl-2 methyl-5 pyrrole

-
C
-
67-47-0
5-hydroxymethyl-2-furfuraldehyde

-
D
-
28564-83-2
2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one

-
E
-
1072-83-9
2-Acetylpyrrole

| Conditions | Yield |
|---|---|
| With L-Lysine hydrochloride; sodium hydrogencarbonate In water at 75℃; for 24h; Product distribution; pH 6.5; other times; |

-
-
121643-36-5
2-Bromo-6-diisopropylamino-1-azafulvene

-
-
74-88-4
methyl iodide

-
A
-
1192-79-6
formyl-2 methyl-5 pyrrole

| Conditions | Yield |
|---|---|
| With tert.-butyl lithium; sodium acetate Yield given. Multistep reaction; |


-
-
1192-79-6
formyl-2 methyl-5 pyrrole

| Conditions | Yield |
|---|---|
| at 220℃; unter vermindertem Druck; |

-
-
1192-79-6
formyl-2 methyl-5 pyrrole

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 75 percent / LiAlH4 / tetrahydrofuran 2: 70 percent / POCl3 View Scheme | |
| Multi-step reaction with 2 steps 1: 74 percent / LiAlH4 / tetrahydrofuran / 36 h / Heating 2: 64.5 percent / POCl3 / 1,2-dichloro-ethane / 1) 0 deg C, 1 h, 2) up to r.t., 3) reflux, 30 min View Scheme | |
| Multi-step reaction with 2 steps 1: 68 percent / H2O / 3 h / Ambient temperature View Scheme | |
| Multi-step reaction with 2 steps 1: 68 percent / H2O / 3 h / Ambient temperature View Scheme |
-
-
67350-50-9
5-(hydroxymethyl)-1H-pyrrole-2-carboxaldehyde

-
-
1192-79-6
formyl-2 methyl-5 pyrrole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 34 percent / N,N'-carbonyldiimidazole; NaH / dimethylformamide / 40 °C 2: H2 / Pd/C View Scheme |
-
-
121643-34-3
N,N-Diisopropylpyrrole-2-formiminium chloride

-
-
1192-79-6
formyl-2 methyl-5 pyrrole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 74 percent / acetonitrile 2: 1) NBS; 2) 0.5 N HCl, NaOH / 1) THF, -78 deg C; 2) MeOH, 20 min 3: -78 °C 4: 2) NaOAc / 1) from -78 deg C to RT; 2) water, 15 h, reflux View Scheme | |
| Multi-step reaction with 4 steps 1: 74 percent / acetonitrile 2: 1) NBS; 2) 0.5 N HCl, NaOH / 1) THF, -78 deg C; 2) MeOH, 20 min 3: t-BuLi / -78 °C 4: 2) NaOAc / 1) from -78 deg C to RT; 2) water, 15 h, reflux View Scheme |

-
-
1192-79-6
formyl-2 methyl-5 pyrrole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: -78 °C 2: 2) NaOAc / 1) from -78 deg C to RT; 2) water, 15 h, reflux View Scheme | |
| Multi-step reaction with 2 steps 1: t-BuLi / -78 °C 2: 2) NaOAc / 1) from -78 deg C to RT; 2) water, 15 h, reflux View Scheme |

-
-
1192-79-6
formyl-2 methyl-5 pyrrole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 1) NBS; 2) 0.5 N HCl, NaOH / 1) THF, -78 deg C; 2) MeOH, 20 min 2: -78 °C 3: 2) NaOAc / 1) from -78 deg C to RT; 2) water, 15 h, reflux View Scheme | |
| Multi-step reaction with 3 steps 1: 1) NBS; 2) 0.5 N HCl, NaOH / 1) THF, -78 deg C; 2) MeOH, 20 min 2: t-BuLi / -78 °C 3: 2) NaOAc / 1) from -78 deg C to RT; 2) water, 15 h, reflux View Scheme |

-
-
1192-79-6
formyl-2 methyl-5 pyrrole

| Conditions | Yield |
|---|---|
| With water; sodium hydrogencarbonate In tetrahydrofuran; pentane at 80℃; for 15h; | 156 mg |
-
-
1192-79-6
formyl-2 methyl-5 pyrrole

-
-
69778-83-2
4-methoxy-3-pyrrolin-2-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In dimethyl sulfoxide at 60℃; for 20h; | 97% |
-
-
1192-79-6
formyl-2 methyl-5 pyrrole

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
276239-45-3
5-methyl-2-formyl-N-(tert-butoxycarbonyl)pyrrole

| Conditions | Yield |
|---|---|
| Stage #1: formyl-2 methyl-5 pyrrole With sodium hydride In tetrahydrofuran at 20℃; for 1h; Metallation; Stage #2: di-tert-butyl dicarbonate In tetrahydrofuran at 20℃; for 1h; Addition; Further stages.; | 95% |
| dmap In acetonitrile at 0 - 25℃; | 94% |
| With dmap In tetrahydrofuran at 0 - 23℃; Inert atmosphere; | 91% |

| Conditions | Yield |
|---|---|
| In xylene for 12h; Heating; | 93% |
-
-
1192-79-6
formyl-2 methyl-5 pyrrole

-
-
19472-74-3
2-(2-bromophenyl)acetonitrile

| Conditions | Yield |
|---|---|
| With potassium phosphate In dimethyl sulfoxide at 130℃; for 24h; Inert atmosphere; | 93% |
-
-
1192-79-6
formyl-2 methyl-5 pyrrole

-
-
74-88-4
methyl iodide

-
-
1193-59-5
1,5-dimethyl-1H-pyrrole-2-carbaldehyde

| Conditions | Yield |
|---|---|
| With potassium hydroxide In N,N-dimethyl-formamide at 25℃; | 90% |

| Conditions | Yield |
|---|---|
| With potassium phosphate In dimethyl sulfoxide at 130℃; for 24h; Inert atmosphere; | 81% |
-
-
1192-79-6
formyl-2 methyl-5 pyrrole

-
-
38622-91-2, 36635-61-7
[(p-methylphenyl)sulfonylmethyl]isonitrile

-
-
179928-16-6
7-methyl-3-tosylpyrrolo[1,2-c]pyrimidine

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran for 2h; Ambient temperature; | 76% |
-
-
1192-79-6
formyl-2 methyl-5 pyrrole

-
-
5697-44-9
p-tolylsulfonylmethyl isocyanide

-
-
179928-16-6
7-methyl-3-tosylpyrrolo[1,2-c]pyrimidine

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran at 20℃; for 12h; Cycloaddition; | 76% |
-
-
1192-79-6
formyl-2 methyl-5 pyrrole

-
-
108-24-7
acetic anhydride

-
-
5971-77-7
4-acetyl-5-methyl-1H-pyrrole-2-carbaldehyde

| Conditions | Yield |
|---|---|
| With tin(IV) chloride In dichloromethane for 8h; Ambient temperature; | 72% |
-
-
1192-79-6
formyl-2 methyl-5 pyrrole

-
-
40400-15-5
2-(2-iodophenyl)acetonitrile

| Conditions | Yield |
|---|---|
| With potassium phosphate In dimethyl sulfoxide at 130℃; for 24h; Inert atmosphere; | 72% |
-
-
1192-79-6
formyl-2 methyl-5 pyrrole

-
-
310430-92-3
1-amino-5-methyl-1H-pyrrole-2-carbonitrile

| Conditions | Yield |
|---|---|
| Stage #1: formyl-2 methyl-5 pyrrole With mesitylenesulfonylhydroxylamine In N,N-dimethyl-formamide Stage #2: With sodium hydride In dichloromethane | 65% |
| Stage #1: formyl-2 methyl-5 pyrrole With mesitylenesulfonylhydroxylamine In dichloromethane for 0.5h; Stage #2: With sodium hydride In DMF (N,N-dimethyl-formamide) at 20℃; for 1.5h; | 65% |
| With potassium hydroxide; hydroxylamine-O-sulfonic acid In water at 0 - 20℃; for 5.5h; | 14% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In diethyl ether for 0.0833333h; | 62% |

| Conditions | Yield |
|---|---|
| With acetic acid at 110℃; for 6h; Inert atmosphere; Sealed tube; Green chemistry; | 57% |

-
-
1192-79-6
formyl-2 methyl-5 pyrrole

-
-
3949-36-8
3-acetylcoumarin

-
-
1217814-66-8
3-(3-(5-methyl-1H-pyrrol-2-yl)acryloyl)-2H-chromen-2-one

| Conditions | Yield |
|---|---|
| With piperidine at 140℃; for 0.0416667h; Microwave irradiation; | 50.1% |
-
-
1192-79-6
formyl-2 methyl-5 pyrrole

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol at 20℃; for 144h; | A n/a B 50% |

-
-
1192-79-6
formyl-2 methyl-5 pyrrole

-
-
336-64-1
bis(heptafluorobutyryl) peroxide

-
-
123825-55-8
3-heptafluoro-5-methylpyrrole-2-carbaldehyde

| Conditions | Yield |
|---|---|
| In 1,1,2-Trichloro-1,2,2-trifluoroethane Ambient temperature; further temperature, further solvent; | 48% |

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 120℃; | 45% |
-
-
1192-79-6
formyl-2 methyl-5 pyrrole

-
-
754-34-7
1,1,1,2,2,3,3-heptafluoro-3-iodo-propane

-
-
123825-55-8
3-heptafluoro-5-methylpyrrole-2-carbaldehyde

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; iron(II) sulfate In dimethyl sulfoxide for 0.5h; Ambient temperature; | 36% |
-
-
1192-79-6
formyl-2 methyl-5 pyrrole

-
-
1313014-23-1
(8-morpholin-4-yl-2-pyridin-4-yl-imidazo[1,2-b]pyridazin-6-yl)-hydrazine

-
-
1313014-09-3
N-(5-methyl-1H-pyrrol-2-ylmethylene)-N-(8-morpholin-4-yl-2-pyridin-4-yl-imidazo[1,2-b]pyridazin-6-yl)-hydrazine

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol at 20℃; for 3h; | 33% |
-
-
1192-79-6
formyl-2 methyl-5 pyrrole

| Conditions | Yield |
|---|---|
| Stage #1: formyl-2 methyl-5 pyrrole; 6',7'-dihydrospiro[piperidine-4,4'-thieno[3,2-c]pyran] at 20℃; Stage #2: Stage #3: With hydrogenchloride In diethyl ether; dichloromethane at 20℃; for 0.333333h; | 33% |
-
-
1192-79-6
formyl-2 methyl-5 pyrrole

-
-
108-24-7
acetic anhydride

-
-
144147-06-8
1-acetyl-5-methylpyrrole-2-carbaldehyde

| Conditions | Yield |
|---|---|
| With sodium acetate for 2h; Heating; | 31% |

| Conditions | Yield |
|---|---|
| With magnesium sulfate In toluene at 20℃; Inert atmosphere; | 25% |
-
-
1192-79-6
formyl-2 methyl-5 pyrrole

-
-
77-78-1
dimethyl sulfate

-
-
1193-59-5
1,5-dimethyl-1H-pyrrole-2-carbaldehyde

| Conditions | Yield |
|---|---|
| With ethanol; sodium 1.) toluene, heating, 2.) toluene, reflux, 3 h; Multistep reaction; |
1H-Pyrrole-2-carboxaldehyde,5-methyl- Specification
The 1H-Pyrrole-2-carboxaldehyde,5-methyl-, with the CAS registry number 1192-79-6, is also known as 5-Methyl-2-formylpyrrole. This chemical's molecular formula is C6H7NO and molecular weight is 109.1259. What's more, its systematic name is called 5-Methyl-1H-pyrrole-2-carbaldehyde.
Physical properties about this chemical are: (1)ACD/LogP: 1.10; (2)#of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.1; (4)ACD/LogD (pH 7.4): 1.1; (5)ACD/BCF (pH 5.5): 4.05; (6)ACD/BCF (pH 7.4): 4.05; (7)ACD/KOC (pH 5.5): 94.7; (8)ACD/KOC (pH 7.4): 94.7; (9)#H bond acceptors: 2; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 22 Å2; (13)Index of Refraction: 1.589; (14)Molar Refractivity: 32.26 cm3; (15)Molar Volume: 95.6 cm3; (16)Polarizability: 12.79×10-24 cm3; (17)Surface Tension: 46.2 dyne/cm; (18)Density: 1.14 g/cm3; (19)Flash Point: 95.8 °C; (20)Enthalpy of Vaporization: 46.26 kJ/mol; (21)Boiling Point: 226.1 °C at 760 mmHg; (22)Vapour Pressure: 0.0835 mmHg at 25 °C.
Preparation of 1H-Pyrrole-2-carboxaldehyde,5-methyl-: this chemical can be prepared by 2-Methyl-pyrrole and N,N-Dimethyl-formamide.
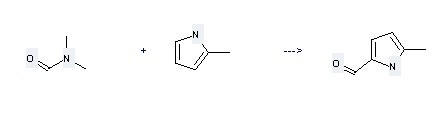
This reaction needs reagent POCl3 and solvent 1,2-Dichloro-ethane at temperature of 0 °C. The reaction time is 1 hour. The yield is 64.5%.
Uses of 1H-Pyrrole-2-carboxaldehyde,5-methyl-: it is used to produce other chemicals. For example, it is used to produce 1-Acetyl-5-methylpyrrole-2-carbaldehyde.
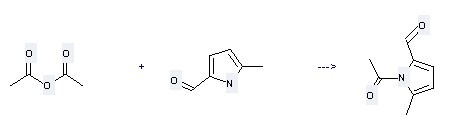
The reaction occurs with reagent Sodium acetate and other condition of heating for 2 hours. The yield is 31%.
You can still convert the following datas into molecular structure:
(1) SMILES: O=Cc1ccc(n1)C
(2) InChI: InChI=1/C6H7NO/c1-5-2-3-6(4-8)7-5/h2-4,7H,1H3
(3) InChIKey: LFWLUDLUCDRDAF-UHFFFAOYAU
Related Products
- 1H-Pyrrole-2-carboxaldehyde, 1-methyl-, 4-(p-chlorophenyl)thiosemicarbazone
- 1H-Pyrrole-2-carboxaldehyde, 1-methyl-, 4-(p-methoxyphenyl)semicarbazone
- 1H-Pyrrole-2-carboxaldehyde, 1-methyl-, 4-phenylsemicarbazone
- 1H-Pyrrole-2-carboxaldehyde, 4-(p-chlorophenyl)semicarbazone
- 1H-Pyrrole-2-carboxaldehyde, 4-phenylsemicarbazone
- 1H-Pyrrole-2-carboxaldehyde, 4-phenylthiosemicarbazone
- 1H-PYRROLE-2-CARBOXALDEHYDE, THIOSEMICARBAZONE
- 1H-Pyrrole-2-carboxaldehyde,1-(4-chlorophenyl)-
- 1H-Pyrrole-2-carboxaldehyde,1-(4-methylphenyl)-
- 1H-Pyrrole-2-carboxaldehyde,4-(4-fluorobenzoyl)-1-methyl-
- 1192-80-9
- 1192-81-0
- 1192845-62-7
- 1192845-63-8
- 119285-07-3
- 119-28-8
- 119290-61-8
- 119291-22-4
- 119299-02-4
- 1193-00-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View