-
Name
2,3,4,6-Tetra-O-acetyl-alpha-D-galactopyranosyl bromide
- EINECS 221-324-0
- CAS No. 3068-32-4
- Article Data325
- CAS DataBase
- Density 1.49 g/cm3
- Solubility Sparingly soluble in water
- Melting Point 83-85 °C
- Formula C14H19BrO9
- Boiling Point 412 °C at 760 mmHg
- Molecular Weight 411.203
- Flash Point 203 °C
- Transport Information
- Appearance White crystalline solid
- Safety 26-36-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Galactopyranosylbromide, tetraacetate, a-D- (6CI,7CI,8CI);a-D-Galactopyranosyl bromide, tetraacetate (9CI);1-a-Bromo-2,3,4,6-tetraacetyl-D-galactopyranose;2,3,4,6-Tetra-O-acetyl-a-D-galactopyranosyl bromide;2,3,4,6-Tetra-O-acetyl-a-D-galactosyl bromide;2,3,4,6-Tetraacetyl-a-D-galactopyranosyl bromide;2,3,4,6-Tetraacetyl-a-D-galactosyl bromide;Acetobromo-a-D-galactose;Acetylbromogalactose;Tetra-O-acetyl-a-D-galactopyranosyl bromide;a-Acetobromogalactose;a-Bromotetraacetylgalactose;a-D-Acetobromogalactose;2,3,4,6-Tetra-O-acetyl-alpha-D-galactopyranosyl bromide;
- PSA 114.43000
- LogP 0.46440
Synthetic route
-
-
4163-60-4
β-D-galactose peracetate

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With hydrogen bromide; acetic acid at 0 - 20℃; | 100% |
| With hydrogen bromide In dichloromethane | 100% |
| With bismuth(III) bromide; triethyl-bromo-silane In dichloromethane at 20℃; for 3h; | 100% |
-
-
83-87-4, 604-68-2, 604-69-3, 2152-77-4, 4026-35-1, 4163-59-1, 4163-60-4, 4163-61-5, 4163-65-9, 4257-94-7, 4257-96-9, 16299-15-3, 19186-39-1, 19189-55-0, 25878-60-8, 25941-03-1, 32445-48-0, 32445-49-1, 32445-53-7, 32445-54-8, 34685-58-0, 34685-59-1, 43168-42-9, 43168-44-1, 43169-22-8, 43169-25-1, 66966-07-2, 70749-17-6, 80184-01-6, 93221-01-3, 93221-02-4, 99630-88-3, 109215-53-4, 115792-70-6, 115792-73-9, 139894-92-1, 139973-25-4, 144071-49-8, 147648-81-5
penta-O-acetyl-α-D-galactopyranose

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With hydrogen bromide; acetic acid In dichloromethane | 100% |
| With hydrogen bromide; acetic acid at 20℃; for 1.5h; | 99% |
| With hydrogen bromide; acetic acid at 20℃; for 18h; Inert atmosphere; | 95% |
-
-
25878-60-8
D-galactose pentaacetate

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With hydrogen bromide; acetic acid at 0 - 10℃; | 100% |
| With bismuth(III) bromide; trimethylsilyl bromide In dichloromethane Ambient temperature; | 99% |
| With bismuth(III) bromide; trimethylsilyl bromide In dichloromethane at 20℃; | 99% |
-
-
59-23-4
D-Galactose

-
-
64-19-7
acetic acid

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With Acetyl bromide at 20℃; | 100% |
-
-
2872-65-3, 14581-81-8, 17042-40-9, 84380-06-3, 105260-62-6
4-methoxyphenyl 2,3,4,6-tetra-O-acetyl-β-D-galactopyranoside

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With Acetyl bromide; zinc dibromide In dichloromethane at 22℃; for 24h; | 98% |
-
-
10257-28-0
D-Galactose

-
-
108-24-7
acetic anhydride

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| Stage #1: D-Galactose With Acetyl bromide In methanol Stage #2: acetic anhydride With acetic acid | 95% |
| With Acetyl bromide In methanol; acetic acid at 20℃; Darkness; | 90% |
| With phosphorus; perchloric acid; bromine | 81.2% |
-
-
197158-78-4
1,2,3,4,6-penta-O-acetyl-D-galactopyranose

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With hydrogen bromide In dichloromethane; acetic acid at 0 - 20℃; | 95% |

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With iodine(I) bromide In dichloromethane; toluene at 20℃; for 1h; Molecular sieve; Inert atmosphere; | 95% |
-
-
3646-73-9
α-D-galactopyranose

-
-
108-24-7
acetic anhydride

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| Stage #1: α-D-galactopyranose; acetic anhydride With perchloric acid at 5 - 45℃; Inert atmosphere; Stage #2: With phosphorus; bromine at 20 - 30℃; Inert atmosphere; | 94% |
-
-
3947-62-4, 6207-76-7, 10343-06-3, 19235-21-3, 22554-70-7, 22860-22-6, 57884-82-9, 62057-79-8, 70191-05-8, 109525-54-4, 140147-37-1, 47339-09-3
2,3,4,6-tetra-O-acetyl-α/β-D-galactopyranose

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With pyridine; (PhO)3P*Br2 In dichloromethane for 0.166667h; | 90% |
| With tris(2,2'-bipyridyl)ruthenium dichloride; carbon tetrabromide; tetrabutylammomium bromide In N,N-dimethyl-formamide Schlenk technique; Sealed tube; Inert atmosphere; Irradiation; | 86% |
| With hydrogen bromide; acetic anhydride In dichloromethane at 20℃; for 4h; | 85% |
-
-
160227-12-3
4-methoxyphenyl 2,3,6-tri-O-acetyl-4-O-(2,3,4,6-tetra-O-acetyl-β-D-galactopyranosyl)-β-D-glucopyranoside

-
A
-
4753-07-5
2,3,6,2',3',4',6'-hepta-O-acetyl-lactosyl bromide

-
B
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
C
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With Acetyl bromide; zinc dibromide In dichloromethane at 22℃; Yields of byproduct given; | A 82% B n/a C n/a |

-
-
5019-22-7
methyl 2,3,4,6-tetra-O-acetyl-α-D-galactopyranoside

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With acyl bromide; zinc dibromide In dichloromethane for 12h; Heating; | 74% |
| With trimethylsilyl bromide; zinc dibromide In dichloromethane for 24h; Ambient temperature; | 41% |
-
-
7226-48-4, 10357-30-9, 14199-54-3, 14199-55-4, 14199-57-6, 18968-06-4, 32445-64-0, 32475-75-5, 52645-74-6, 67322-02-5, 100018-31-3, 101540-16-3, 140148-47-6
1,2,3,4,6-penta-O-acetyl-β-D-galactopyranoside

-
-
108-24-7
acetic anhydride

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With hydrogen bromide; acetic acid at 20℃; for 3.16667h; Inert atmosphere; | 71% |
-
-
59-23-4
D-Galactose

-
-
108-24-7
acetic anhydride

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With perchloric acid anschl. mit Phosphor, Brom und Wasser; |
-
-
13992-16-0, 23661-28-1, 28512-68-7, 65236-85-3, 86782-39-0, 108032-93-5, 121524-01-4, 121570-34-1, 24404-53-3
1-deoxy-1-(phenylthio)-2,3,4,6-tetra-O-acetyl-β-D-galactopyranose

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With iodine(I) bromide In dichloromethane at 0℃; for 0.166667h; Yield given; |
-
-
55692-87-0
isopropyl 2,3,4,6-tetra-O-acetyl-1-thio-β-D-galactopiranoside

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With iodine(I) bromide In dichloromethane at 0℃; for 0.166667h; Yield given; |
-
-
55722-48-0
methyl 2,3,4,6-tetra-O-acetyl-1-thio-β-D-galactopyranoside

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With iodine(I) bromide In dichloromethane at 0℃; for 0.166667h; Yield given; | |
| With iodine(I) bromide In dichloromethane at 0℃; for 0.166667h; Bromination; |
-
-
10257-28-0
D-Galactose

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: pyridine 2: HBr / acetic acid View Scheme | |
| Multi-step reaction with 2 steps 1: sodium acetate / 2.25 h / 100 °C / air 2: hydrogen bromide; acetic acid / 3.17 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: pyridine / 20 °C 2: acetic anhydride; hydrogen bromide; acetic acid / dichloromethane / 20 °C View Scheme |
-
-
3616-19-1, 5328-50-7, 5346-90-7, 6291-42-5, 6920-00-9, 20764-63-0, 20880-60-8, 20880-65-3, 22352-19-8, 23973-20-8, 32447-69-1, 34213-32-6, 52554-32-2, 53956-64-2, 56907-29-0, 56994-12-8, 56994-13-9, 57128-38-8, 67650-36-6, 68036-17-9, 68036-19-1, 78215-50-6, 78215-51-7, 79549-48-7, 80446-82-8, 85249-04-3, 93221-83-1, 93779-27-2, 111407-83-1, 111407-84-2, 111407-85-3, 120328-65-6, 123809-59-6, 132341-46-9, 132341-47-0, 132341-49-2, 145307-94-4
1',2',3',6',2,3,4,6-octa-O-acetyl-β-D-lactose

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 90 percent / BF3*Et2O / CH2Cl2 2: AcBr, ZnBr2 / CH2Cl2 / 22 °C View Scheme |
-
-
1138026-31-9
2,3,4,6-tetra-O-acetyl-β-D-galactopyranosyl-1-C-sulfonamide

-
A
-
1138439-62-9
C14H20BrNO11S

-
B
-
1138439-63-0
C14H20BrNO11S

-
C
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With bromine; potassium carbonate In chloroform for 4h; Reflux; Heat lamp; |

-
-
3646-73-9
α-D-galactopyranose

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: pyridine / 20 h / 20 °C / Inert atmosphere 2: tert-butyl alcohol; hydrogen bromide / acetic acid / 0 - 20 °C View Scheme |
-
-
7296-64-2
β-D-galactopyranoside

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: pyridine / 24 h / 20 °C 2: acetic anhydride; hydrogen bromide; acetic acid / dichloromethane / 24 h / 0 - 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: pyridine 2: hydrogen bromide; acetic acid / dichloromethane / 3 h / 20 °C View Scheme |
-
-
25878-60-8
D-galactose pentaacetate

-
A
-
572-09-8, 3068-32-4, 4026-32-8, 6919-96-6, 13242-53-0, 14227-54-4, 14795-18-7, 19186-59-5, 21698-13-5, 29907-26-4, 53369-42-9, 60619-58-1, 61303-35-3, 67337-79-5, 69127-37-3, 70749-15-4, 72002-90-5, 75247-30-2, 75880-74-9, 78418-57-2, 102339-89-9, 102339-90-2, 103530-40-1, 114028-03-4, 114718-45-5, 138514-70-2, 138514-71-3, 19285-38-2
acetobromogalactose

-
B
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With hydrogen bromide; acetic acid at 4℃; |
-
-
7512-17-6, 72-87-7, 1136-42-1, 1136-44-3, 6082-29-7, 6730-06-9, 7772-94-3, 10036-64-3, 14131-60-3, 14131-64-7, 14131-68-1, 14215-68-0, 62057-49-2, 62057-50-5, 62057-51-6, 62057-52-7, 62057-53-8, 62057-54-9, 62057-55-0, 62057-56-1, 62075-50-7, 62137-54-6, 134451-97-1, 134522-12-6, 148347-16-4
N-Acetyl-D-glucosamine

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 20 °C 2: acetic anhydride; hydrogen bromide; acetic acid / dichloromethane / 20 °C View Scheme |
-
-
70191-05-8
2,3,4,6-tetra-O-acetyl-β-D-galactopyranose

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With hydrogen bromide; acetic acid In dichloromethane at 20℃; for 3h; |
-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

-
-
13992-26-2
(2R,3S,4S,5R,6R)-2-(acetoxymethyl)-6-azidotetrahydro-2H-pyran-3,4,5-triyl triacetate

| Conditions | Yield |
|---|---|
| With sodium azide; tetra(n-butyl)ammonium hydrogensulfate; sodium hydrogencarbonate In dichloromethane | 100% |
| With sodium azide; tetra(n-butyl)ammonium hydrogensulfate; sodium hydrogencarbonate In dichloromethane; water at 20℃; | 100% |
| With tetramethylguanidinum azide In nitromethane at 25℃; for 4h; | 99% |
-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With silver perchlorate; N-ethyl-N,N-diisopropylamine In benzene at 20℃; for 1h; Molecular sieve; Inert atmosphere; Darkness; | 100% |
| With N-ethyl-N,N-diisopropylamine; silver(l) oxide In benzene for 6h; Heating; |
-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

-
-
724733-04-4
4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,11-heptadecafluoro-undecanoic acid (4-mercapto-phenyl)-methyl-amide

| Conditions | Yield |
|---|---|
| With sodium carbonate In water; ethyl acetate at 20℃; for 2h; | 100% |
-
-
501-36-0
(E)-5-[2-4-(hydroxyphenyl)ethenyl]-1,3-benzenediol

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With 2,4,6-trimethyl-pyridine; silver carbonate; molecular sieve, 3 A In dichloromethane; acetonitrile at 25℃; for 72h; Under anhydrous N2; | 100% |
-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

-
-
13121-62-5
2,3,4,6-tetra-O-acetyl-1,5-anhydro-D-galactitol

| Conditions | Yield |
|---|---|
| With Cp2Ti(BH4) In tetrahydrofuran for 0.75h; Ambient temperature; | 99% |
| With 2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride In toluene for 4h; Reflux; | 97% |
| With hydrogen; triethylamine; palladium on activated charcoal In ethyl acetate at 20℃; under 760.051 Torr; for 3h; | 96% |

| Conditions | Yield |
|---|---|
| With 2,4,6-trimethyl-pyridine; 4 A molecular sieve; mercury dibromide In nitromethane; chloroform; benzene for 22h; Ambient temperature; | 99% |
-
-
88-30-2
3-trifluoromethyl-4-nitrophenol

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide In dichloromethane; water at 50℃; for 1h; pH=8 - 9; | 99% |
-
-
400-99-7
2-nitro-4-trifluoromethylphenol

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide In dichloromethane; water at 50℃; for 1h; pH=8 - 9; | 99% |
-
-
403-19-0
2-fluoro-4-nitrophenol

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide In dichloromethane; water at 50℃; for 1h; pH=8 - 9; | 99% |
| With sodium hydroxide; tetrabutylammomium bromide In dichloromethane at 35℃; for 4h; | 77% |
| With tetrabutylammomium bromide; sodium hydroxide |

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol for 18h; | 99% |

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 18℃; for 12h; Inert atmosphere; regioselective reaction; | 99% |
| With potassium carbonate In N,N-dimethyl-formamide at 25℃; for 12h; Inert atmosphere; | |
| With potassium carbonate In N,N-dimethyl-formamide at 25℃; for 12h; Inert atmosphere; |
-
-
67-56-1
methanol

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

-
-
5019-23-8
methyl 2,3,4,6-tetra-O-acetyl-β-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With triethylamine; 1-butyl-3-methylimidazolium trifluoromethanesulfonimide at 42℃; for 1h; Kinetics; Reagent/catalyst; Temperature; | 98% |
| With 4 A molecular sieve; iodine for 1h; Ambient temperature; | 95% |
| With silver carbonate | |
| With phenylmercuric acetate |
-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

-
-
4098-06-0
triacetyl-D-galactal

| Conditions | Yield |
|---|---|
| With edetate disodium; chromium(II) acetate In water; ethyl acetate for 18h; Ambient temperature; | 98% |
| With tetrakis(acetato)dichromium(II) dihydrate; edetate disodium In water; ethyl acetate for 18h; pH=5.0; | 97% |
| With β‐cyclodextrin; zinc In water at 20℃; for 0.5h; Sonication; | 93% |
-
-
18678-94-9
2-benzyloxy-3-hexadecyloxy-1-propanol

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

-
-
82002-26-4
Acetic acid (2R,3S,4S,5R,6R)-3,5-diacetoxy-2-acetoxymethyl-6-(2-benzyloxy-3-hexadecyloxy-propoxy)-tetrahydro-pyran-4-yl ester

| Conditions | Yield |
|---|---|
| With silver(l) oxide | 98% |
-
-
108-98-5
thiophenol

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

-
-
13992-16-0, 23661-28-1, 28512-68-7, 65236-85-3, 86782-39-0, 108032-93-5, 121524-01-4, 121570-34-1, 24404-53-3
1-deoxy-1-(phenylthio)-2,3,4,6-tetra-O-acetyl-β-D-galactopyranose

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetrabutylammomium bromide In benzene for 0.5h; Ambient temperature; | 98% |
| With potassium carbonate for 0.5h; Neat (no solvent); Ball milling; | 95% |
| With triethylamine In acetonitrile for 1h; Ambient temperature; | 82% |
| With tetra(n-butyl)ammonium hydrogensulfate; sodium carbonate In ethyl acetate | 75% |
| With tetrabutylammomium bromide; sodium carbonate In dichloromethane; water at 20℃; for 4h; stereoselective reaction; |
-
-
92841-84-4
allyl 2-acetamido-2-deoxy-4,6-O-benzylidene-α-D-galactopyranoside

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

-
-
155739-81-4
(2,3,4,6-tetra-O-acetyl-α-D-galactopyranosyl)-(1-3)-2-acetamido-4,6-O-benzylidene-2-deoxy-α-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With mercury(II) cyanide In nitromethane; benzene at 20℃; for 18h; | 98% |
| In nitromethane; benzene at 25℃; | 78% |
| With mercury(II) cyanide In nitromethane; benzene at 50℃; | 65% |
-
-
97-51-8
5-Nitrosalicylaldehyde

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With silver(l) oxide In acetonitrile for 4h; Darkness; | 98% |
| With silver(l) oxide In acetonitrile for 4h; Darkness; | 98% |
| With silver(l) oxide In acetonitrile at 20℃; for 22.5h; Inert atmosphere; | 87% |
-
-
1824-94-8
methyl beta-D-galactopyranoside

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

-
-
187458-88-4
methyl 3-O-(2’,3’,4’,6’-tetra-O-acetyl-β-D-galactopyranosyl)-β-D-galactopyranoside

| Conditions | Yield |
|---|---|
| Stage #1: methyl beta-D-galactopyranoside With diphenyltin(IV) dichloride In tetrahydrofuran at 20℃; for 0.166667h; Stage #2: 1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside With 5,5'-dimethyl-2,2'-bipyridine; silver(l) oxide In tetrahydrofuran at 20 - 45℃; for 24h; Koenigs-Knorr Glycosidation; stereoselective reaction; | 98% |
-
-
5115-14-0
monobenzyldithiocarbamate sodium

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

-
-
1590413-29-8
S-(2,3,4,6-tetra-O-acetyl-β-D-galactopyranosyl)-N-benzyl dithiocarbamate

| Conditions | Yield |
|---|---|
| In acetone at 20℃; for 2h; | 98% |
-
-
108-93-0
cyclohexanol

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

-
-
54656-59-6
(2R,3S,4S,5R,6R)-2-(acetoxymethyl)-6-(cyclohexyloxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate

| Conditions | Yield |
|---|---|
| With silver carbonate In dichloromethane | 97% |
-
-
131242-36-9
2-sulfanylpyrimidine

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

-
A
-
171973-23-2
Acetic acid (2S,3R,4S,5S,6R)-4,5-diacetoxy-6-acetoxymethyl-2-(pyrimidin-2-ylsulfanyl)-tetrahydro-pyran-3-yl ester

-
B
-
15054-61-2
2,2'-(methylenebis(thio))bis(pyrimidine)

-
C
-
19834-93-6
chloro(pyrimidin-2-ylthio)methane

| Conditions | Yield |
|---|---|
| With tetra(n-butyl)ammonium hydrogensulfate; sodium carbonate In dichloromethane for 1h; Ambient temperature; | A 97% B n/a C n/a |

-
-
106-45-6
para-thiocresol

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

-
-
28244-94-2, 28244-99-7, 86782-41-4, 131488-65-8
4-methylphenyl 2,3,4,6-tetra-O-acetyl-1-thio-β-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With potassium carbonate for 0.5h; Neat (no solvent); Ball milling; | 97% |
| With bismuth subcarbonate In neat (no solvent) for 2h; Molecular sieve; Milling; | 88% |
| With tetrabutylammomium bromide; sodium carbonate In dichloromethane; water at 20℃; for 4h; stereoselective reaction; |
-
-
17356-08-0
thiourea

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

-
-
84062-53-3
2,3,4,6-tetra-O-acetylgalactopyranosyl-1-β-pseudothiourea hydrobromide

| Conditions | Yield |
|---|---|
| In acetone at 70℃; for 16h; | 96% |
| With isopropyl alcohol |
-
-
14687-15-1
methyl L-fucopyranoside

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With arylboronic promoter; tetraethylammonium iodide; silver carbonate; 4 A molecular sieve In tetrahydrofuran at 0 - 50℃; | 96% |
| With [2-(1-methyl-1H-imidazol-2-yl)phenyl]boronic acid; silver(l) oxide In acetonitrile at 30℃; for 24h; Koenigs-Knorr Glycosidation; Inert atmosphere; regioselective reaction; | 93% |
-
-
40889-91-6
2-chloro-5-(trifluoromethyl)phenol

-
-
3068-32-4
1-bromo-1-deoxy-2,3,4,6-tetra-O-acetyl-a-D-galactopyranoside

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide In dichloromethane; water at 50℃; for 1h; pH=8 - 9; | 96% |
2,3,4,6-Tetra-O-acetyl-alpha-D-galactopyranosyl bromide Chemical Properties
Product Name: Tetra-O-acetyl-alpha-D-galactopyranosyl bromide (CAS NO.3068-32-4)
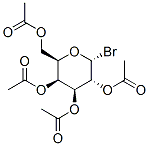
Molecular Formula: C14H19BrO9
Molecular Weight: 411.2g/mol
Mol File: 3068-32-4.mol
Melting Point: 83-85 °C
Boiling point: 412 °C at 760 mmHg
Storage Temperature: -20°C
Flash Point: 203 °C
Density: 1.49 g/cm3
Water Solubility: Sparingly soluble
Surface Tension: 47.9 dyne/cm
Enthalpy of Vaporization: 66.46 kJ/mol
Vapour Pressure: 5.35E-07 mmHg at 25°C
XLogP3-AA: 1.5
H-Bond Donor: 0
H-Bond Acceptor: 9
Structure Descriptors of Tetra-O-acetyl-alpha-D-galactopyranosyl bromide (CAS NO.3068-32-4):
IUPAC Name: [4,5-diacetyloxy-2-(acetyloxymethyl)-6-bromooxan-3-yl] acetate
Canonical SMILES: CC(=O)OCC1C(C(C(C(O1)Br)OC(=O)C)OC(=O)C)OC(=O)C
InChI: InChI=1S/C14H19BrO9/c1-6(16)20-5-10-11(21-7(2)17)12(22-8(3)18)13(14(15)24-10)23-9(4)19/h10-14H,5H2,1-4H3
InChIKey: CYAYKKUWALRRPA-UHFFFAOYSA-N
Product Categories: Sugars, Carbohydrates & Glucosides; 13C & 2H Sugars; Carbohydrates & Derivatives
2,3,4,6-Tetra-O-acetyl-alpha-D-galactopyranosyl bromide Safety Profile
Safety Information of Tetra-O-acetyl-alpha-D-galactopyranosyl bromide (CAS NO.3068-32-4):
Hazard Codes: Xi
Risk Statements: 36/37/38
36: Irritating to the eyes
37: Irritating to the respiratory system
38: Irritating to the skin
Safety Statements: 26-36-37/39
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
37: Wear suitable gloves
39: Wear eye/face protection
WGK Germany: 3
F: 8-10-21
2,3,4,6-Tetra-O-acetyl-alpha-D-galactopyranosyl bromide Specification
Tetra-O-acetyl-alpha-D-galactopyranosyl bromide , its CAS NO. is 3068-32-4, the synonyms are EINECS 221-324-0 ; alpha-D-Galactopyranosyl bromide, tetraacetate .
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 30683-38-6
- 3068-34-6
- 3068-39-1
- 30685-43-9
- 3068-76-6
- 3068-78-8
- 30688-66-5
- 3068-88-0
- 306-91-2
- 3069-19-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View