-
Name
2,4-Pentadienoic acid
- EINECS 210-976-1
- CAS No. 626-99-3
- Article Data20
- CAS DataBase
- Density 1.039 g/cm3
- Solubility
- Melting Point 69-72 °C
- Formula C5H6O2
- Boiling Point 215 °C at 760 mmHg
- Molecular Weight 98.1014
- Flash Point 121.5 °C
- Transport Information
- Appearance
- Safety 22-24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms a,g-Pentadienoic acid (3CI);1,3-Butadiene-1-carboxylicacid;1-Carboxy-1,3-butadiene;1-Carboxybutadiene;Butadiene-1-carboxylic acid;NSC 16628;β-Vinylacrylic acid;
- PSA 37.30000
- LogP 0.81320
Synthetic route

| Conditions | Yield |
|---|---|
| in aetherischer Loesung; |


-
-
626-99-3
1,3-butadiene-1-carboxylic acid

| Conditions | Yield |
|---|---|
| bei der Kalischmelze; |
-
-
13038-12-5
ethyl 2,4-pentadienoate

-
-
626-99-3
1,3-butadiene-1-carboxylic acid

| Conditions | Yield |
|---|---|
| (hydrolysis); |
-
-
14403-19-1
(RS)-3-amino-pent-4-enoic acid

-
-
626-99-3
1,3-butadiene-1-carboxylic acid

| Conditions | Yield |
|---|---|
| (heating); |

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)nickel (0); Maleinsaeureanhydrid; 1,2-bis-(dicyclohexylphosphino)ethane 1.) THF, RT, 48 h, 2.) THF, 20 deg C, 48 h; Yield given. Multistep reaction; |

-
-
626-99-3
1,3-butadiene-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide |

-
-
626-99-3
1,3-butadiene-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide |
-
-
14403-16-8
1-benzoyl-4-vinyl-azetidin-2-one

-
-
626-99-3
1,3-butadiene-1-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: (i) NaOH, (ii) aq. HCl 2: (heating) View Scheme |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In water at 39.84℃; Temperature; Reagent/catalyst; |

-
-
626-99-3
1,3-butadiene-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With 4-hydroxypent-2-enoate dehydratase Enzymatic reaction; |

-
-
626-99-3
1,3-butadiene-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With 2,4-pentadienoyl-CoA hydrolase |
-
-
20406-62-6
2-oxo-4-pentenoic acid

-
-
626-99-3
1,3-butadiene-1-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 2-oxopent-4-enoate reductase / Enzymatic reaction 2: 2-hydroxypent-4-enoate mutase / Enzymatic reaction 3: 3-hydroxypent-4-enoate dehydratase / Enzymatic reaction View Scheme | |
| Multi-step reaction with 3 steps 1: 2-oxopent-4-enoate reductase / Enzymatic reaction 2: 2-hydroxypent-4-enoate vinylisomerase / Enzymatic reaction 3: 5-hydroxypent-2-enoate dehydratase / Enzymatic reaction View Scheme |
-
-
67951-43-3
2-hydroxypent-4-enoic acid

-
-
626-99-3
1,3-butadiene-1-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 2-hydroxypent-4-enoate mutase / Enzymatic reaction 2: 3-hydroxypent-4-enoate dehydratase / Enzymatic reaction View Scheme | |
| Multi-step reaction with 2 steps 1: 2-hydroxypent-4-enoate vinylisomerase / Enzymatic reaction 2: 5-hydroxypent-2-enoate dehydratase / Enzymatic reaction View Scheme |
-
-
23251-44-7
hydroxyvalerenic acid

-
-
626-99-3
1,3-butadiene-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With 5-hydroxypent-2-enoate dehydratase Enzymatic reaction; |
-
-
81357-28-0
(RS)-3-hydroxy-4-pentenoic acid

-
-
626-99-3
1,3-butadiene-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With 3-hydroxypent-4-enoate dehydratase Enzymatic reaction; |
-
-
41453-55-8, 3318-73-8
4-hydroxy-2-oxopentanoic acid

-
-
626-99-3
1,3-butadiene-1-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 4-hydroxy-2-oxovalerate 3-dehydratase / Enzymatic reaction 2: 2-oxopent-3-enoate reductase / Enzymatic reaction 3: 2-hydroxypent-3-enoate dehydratase/vinylisomerase / Enzymatic reaction View Scheme | |
| Multi-step reaction with 4 steps 1: 4-hydroxy 2- oxovalerate dehydratase / Enzymatic reaction 2: 2-oxopent-4-enoate reductase / Enzymatic reaction 3: 2-hydroxypent-4-enoate mutase / Enzymatic reaction 4: 3-hydroxypent-4-enoate dehydratase / Enzymatic reaction View Scheme | |
| Multi-step reaction with 4 steps 1: 4-hydroxy 2- oxovalerate dehydratase / Enzymatic reaction 2: 2-oxopent-4-enoate reductase / Enzymatic reaction 3: 2-hydroxypent-4-enoate vinylisomerase / Enzymatic reaction 4: 5-hydroxypent-2-enoate dehydratase / Enzymatic reaction View Scheme | |
| Multi-step reaction with 4 steps 1: 4-hydroxy-2-oxovalerate 3-dehydratase / Enzymatic reaction 2: 2-oxopent-3-enoate reductase / Enzymatic reaction 3: 2-hydroxypent-3-enoate vinylisomerase / Enzymatic reaction 4: 4-hydroxypent-2-enoate dehydratase / Enzymatic reaction View Scheme |

-
-
626-99-3
1,3-butadiene-1-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 2-oxopent-3-enoate reductase / Enzymatic reaction 2: 2-hydroxypent-3-enoate dehydratase/vinylisomerase / Enzymatic reaction View Scheme | |
| Multi-step reaction with 3 steps 1: 2-oxopent-3-enoate reductase / Enzymatic reaction 2: 2-hydroxypent-3-enoate vinylisomerase / Enzymatic reaction 3: 4-hydroxypent-2-enoate dehydratase / Enzymatic reaction View Scheme |

-
-
626-99-3
1,3-butadiene-1-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 3- oxo-4-hydroxypentanoate reductase / Enzymatic reaction 2: 3,4-dihydroxypentanoate dehydratase / Enzymatic reaction 3: 4-hydroxypent-2-enoate dehydratase / Enzymatic reaction View Scheme |
-
-
58957-59-8
3,4-dihydroxy-valeric acid

-
-
626-99-3
1,3-butadiene-1-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 3,4-dihydroxypentanoate dehydratase / Enzymatic reaction 2: 4-hydroxypent-2-enoate dehydratase / Enzymatic reaction View Scheme |

-
-
626-99-3
1,3-butadiene-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With 2-hydroxypent-3-enoate dehydratase/vinylisomerase Enzymatic reaction; | |
| Multi-step reaction with 2 steps 1: 2-hydroxypent-3-enoate vinylisomerase / Enzymatic reaction 2: 4-hydroxypent-2-enoate dehydratase / Enzymatic reaction View Scheme |

| Conditions | Yield |
|---|---|
| With 4-(dimethylamino)pyridine N-oxide; 3,5-bis-trifluromethylphenylboronic acid In fluorobenzene for 16h; Reflux; chemoselective reaction; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-butadiene-1-carboxylic acid With triethylamine; methyl chloroformate In diethyl ether at 0℃; for 0.5h; Stage #2: With sodium tetrahydroborate In methanol; diethyl ether at 0℃; for 0.5h; | 95% |
| With lithium aluminium tetrahydride |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0℃; | 94% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 95℃; under 750.075 Torr; for 0.783333h; Diels-Alder reaction; | 75% |
-
-
626-99-3
1,3-butadiene-1-carboxylic acid

-
-
100-51-6
benzyl alcohol

-
-
380870-40-6
benzyl trans-2,4-pentadienoate

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 5.5h; Cooling with ice; | 71% |

| Conditions | Yield |
|---|---|
| With 3,5-bis-trifluromethylphenylboronic acid In fluorobenzene for 16h; Reflux; chemoselective reaction; | A 45% B n/a |

-
-
626-99-3
1,3-butadiene-1-carboxylic acid

-
-
106-51-4
p-benzoquinone

-
-
6943-52-8, 93601-99-1, 93602-00-7
5,8-dioxo-1,4,4a,5,8,8a-hexahydronaphthalene-1-carboxylic acid

| Conditions | Yield |
|---|---|
| In toluene at 80℃; for 24h; Diels-Alder Cycloaddition; | 24% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride | 20% |
| With sulfuric acid | |
| With sulfuric acid Heating; |

| Conditions | Yield |
|---|---|
| With acetic acid; hydroquinone at 100℃; |

| Conditions | Yield |
|---|---|
| With propionic acid; hydroquinone |

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
626-99-3
1,3-butadiene-1-carboxylic acid

-
-
85858-56-6
4,5-dibromo-pent-2-enoic acid

| Conditions | Yield |
|---|---|
| With carbon disulfide |
-
-
626-99-3
1,3-butadiene-1-carboxylic acid

-
-
16666-42-5
(Z)-2-pentenoic acid

| Conditions | Yield |
|---|---|
| With methanol; platinum(IV) oxide; hydrogen under 1471.02 Torr; Hydrogenation; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide bei der elektrolytischen Reduktion an einer Kupferkathode; | |
| With sodium amalgam; sodium hydrogencarbonate; sodium carbonate | |
| With ammonia; lithium |
-
-
626-99-3
1,3-butadiene-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With water; hypochloric acid substance of ingold,Prichard,Smith; |

| Conditions | Yield |
|---|---|
| With potassium permanganate at 0℃; |
-
-
626-99-3
1,3-butadiene-1-carboxylic acid

-
-
52126-35-9
trans 2,4-pentadienoyl chloride

| Conditions | Yield |
|---|---|
| With thionyl chloride; zinc(II) chloride; Petroleum ether; zinc at 60℃; |

| Conditions | Yield |
|---|---|
| With sodium amalgam |
-
-
626-99-3
1,3-butadiene-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With water; hypochloric acid at 50℃; |
-
-
626-99-3
1,3-butadiene-1-carboxylic acid

-
-
78508-86-8
(E)-5-bromo-4-hydroxy-2-pentenoic acid

| Conditions | Yield |
|---|---|
| With diethyl ether; water; hypobromous acid | |
| With water; hypobromous acid |
-
-
626-99-3
1,3-butadiene-1-carboxylic acid

| Conditions | Yield |
|---|---|
| With chloroform; chlorine |
-
-
626-99-3
1,3-butadiene-1-carboxylic acid

-
-
135038-94-7
2,3,4,5-tetrabromo-valeric acid

| Conditions | Yield |
|---|---|
| With carbon disulfide; bromine | |
| With bromine |

| Conditions | Yield |
|---|---|
| With sodium hydroxide bei der elektrolytischen Reduktion; |
-
-
626-99-3
1,3-butadiene-1-carboxylic acid

-
-
2777-25-5
dinitromethylene-methoxy-amine oxide

-
-
17823-17-5
3-(2-methoxy-3,3-dinitro-isoxazolidin-5-yl)-acrylic acid

| Conditions | Yield |
|---|---|
| In toluene at 0℃; |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride at -30℃; |
-
-
626-99-3
1,3-butadiene-1-carboxylic acid

-
-
60053-24-9
penta-3,4-dienoic acid

| Conditions | Yield |
|---|---|
| In diethyl ether Irradiation; |
2,4-Pentadienoic acid Specification
The 2,4-Pentadienoic acid, with the CAS registry number 626-99-3, is also known as 3,3'-Dinitrobenzidine. Its EINECS number is 210-976-1. This chemical's molecular formula is C5H6O2 and molecular weight is 98.1. What's more, its IUPAC name is (2E)-penta-2,4-dienoic acid. You should not breathe dust. When using it, you must avoid contacting with skin and eyes. It is stable at common pressure and temperature, and it should be sealed and stored at the temperature of -20 °C.
Physical properties of 2,4-Pentadienoic acid are: (1)ACD/LogP: 0.82; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -0.25; (4)ACD/LogD (pH 7.4): -2.05; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 5.69; (8)ACD/KOC (pH 7.4): 1; (9)#H bond acceptors: 2; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 26.3 Å2; (13)Index of Refraction: 1.474; (14)Molar Refractivity: 26.56 cm3; (15)Molar Volume: 94.4 cm3; (16)Polarizability: 10.52×10-24cm3; (17)Surface Tension: 34.6 dyne/cm; (18)Density: 1.039 g/cm3; (19)Flash Point: 121.5 °C; (20)Enthalpy of Vaporization: 49.73 kJ/mol; (21)Boiling Point: 215 °C at 760 mmHg; (22)Vapour Pressure: 0.0586 mmHg at 25°C.
Uses of 2,4-Pentadienoic acid: it can be used to produce penta-2,4-dienoic acid methyl ester by heating. It will need reagent H2SO4.
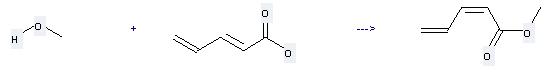
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: C=CC=CC(=O)O
(2)Isomeric SMILES: C=C/C=C/C(=O)O
(3)InChI: InChI=1S/C5H6O2/c1-2-3-4-5(6)7/h2-4H,1H2,(H,6,7)/b4-3+
(4)InChIKey: SDVVLIIVFBKBMG-ONEGZZNKSA-N
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 6270-06-0
- 62700-69-0
- 6270-07-1
- 627-00-9
- 627-01-0
- 6270-11-7
- 6270-12-8
- 6270-13-9
- 6270-14-0
- 627-02-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View