-
Name
2,4-Pyridinedicarboxylic acid
- EINECS 207-892-2
- CAS No. 499-80-9
- Article Data23
- CAS DataBase
- Density 1.551 g/cm3
- Solubility Soluble in water at 20°C 4.5 g/L.
- Melting Point 243-246 °C
- Formula C7H5NO4
- Boiling Point 574.8 °C at 760 mmHg
- Molecular Weight 167.121
- Flash Point 301.4 °C
- Transport Information
- Appearance white to off-white crystalline powder
- Safety 24/25-26
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2,4-Dicarboxylpyridine;2,4-Lutidinic acid;Lutidinic acid;NSC 403248;
- PSA 87.49000
- LogP 0.47800
Synthetic route

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; potassium tert-butylate; oxygen In 1,2-dimethoxyethane at 60℃; under 3800 Torr; for 48h; | 52% |
| With potassium permanganate | |
| With permanganate(VII) ion |

| Conditions | Yield |
|---|---|
| With permanganate(VII) ion |

| Conditions | Yield |
|---|---|
| With permanganate(VII) ion |

-
-
499-80-9
pyridine-2,4-dicarboxylic acid

| Conditions | Yield |
|---|---|
| With permanganate(VII) ion |

-
-
499-80-9
pyridine-2,4-dicarboxylic acid

| Conditions | Yield |
|---|---|
| With alkaline permanganate solution |

-
-
499-80-9
pyridine-2,4-dicarboxylic acid

| Conditions | Yield |
|---|---|
| With permanganate(VII) ion |
-
-
108-47-4
2,4-lutidine

-
-
7664-93-9
sulfuric acid

-
A
-
4021-11-8
2-methylisonicotinic acid

-
B
-
499-80-9
pyridine-2,4-dicarboxylic acid

| Conditions | Yield |
|---|---|
| at 300℃; |

-
-
54089-05-3
2-carbamoyl-4-cyanopyridine

-
-
499-80-9
pyridine-2,4-dicarboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water |

| Conditions | Yield |
|---|---|
| With thiostrepton; ammonium chloride at 30℃; for 168h; | |
| With disodium hydrogenphosphate; potassium dihydrogenphosphate; chloramphenicol; magnesium sulfate; ammonium chloride; thiostrepton A; sodium chloride; calcium chloride In water at 30℃; for 168h; Reagent/catalyst; Enzymatic reaction; |

-
-
499-80-9
pyridine-2,4-dicarboxylic acid

| Conditions | Yield |
|---|---|
| With thiostrepton; ammonium chloride at 30℃; for 168h; Time; |

-
-
499-80-9
pyridine-2,4-dicarboxylic acid

| Conditions | Yield |
|---|---|
| With thiostrepton; ammonium chloride at 30℃; for 96h; | |
| With disodium hydrogenphosphate; potassium dihydrogenphosphate; chloramphenicol; magnesium sulfate; ammonium chloride; thiostrepton A; sodium chloride; calcium chloride In water at 30℃; for 96h; Flow reactor; Enzymatic reaction; |

-
-
499-80-9
pyridine-2,4-dicarboxylic acid

| Conditions | Yield |
|---|---|
| With disodium hydrogenphosphate; potassium dihydrogenphosphate; chloramphenicol; magnesium sulfate; ammonium chloride; thiostrepton A; sodium chloride; calcium chloride In water at 30℃; for 216h; Flow reactor; Enzymatic reaction; |
-
-
499-80-9
pyridine-2,4-dicarboxylic acid

-
-
64-17-5
ethanol

-
-
41438-38-4
diethyl pyridine-2,4-dicarboxylate

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid for 24h; Heating; | 100% |
| With toluene-4-sulfonic acid In toluene at 110℃; | 88% |
| Stage #1: ethanol With thionyl chloride at 0℃; for 0.5h; Inert atmosphere; Stage #2: pyridine-2,4-dicarboxylic acid In ethanol for 3h; Inert atmosphere; Reflux; | 82% |
-
-
499-80-9
pyridine-2,4-dicarboxylic acid

-
-
64-17-5
ethanol

-
-
142074-49-5
2-(ethoxycarbonyl)isonicotinic acid

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid at 80℃; Fischer-Speier Esterification; Inert atmosphere; | 100% |
| at 80℃; Acidic conditions; |

| Conditions | Yield |
|---|---|
| In ethanol; water | 99.3% |

-
-
499-80-9
pyridine-2,4-dicarboxylic acid

| Conditions | Yield |
|---|---|
| In water a soln. of acid in water was added to the freshly prepared soln. of complex, the mixt. was stirred at 90°C for 45 min; cooled on ice bath, HPLC analyses; | 99% |
| In water |
-
-
499-80-9
pyridine-2,4-dicarboxylic acid

-
-
7732-18-5
water

-
-
6046-93-1
copper(II) acetate monohydrate

-
-
363176-04-9, 831196-27-1
[Cu(pyridine-2,4-dicarboxylic acid(-1H))2(H2O)2]

| Conditions | Yield |
|---|---|
| In water 70°C; elem. anal.; | 99% |


| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; | 99% |
-
-
67-56-1
methanol

-
-
499-80-9
pyridine-2,4-dicarboxylic acid

-
-
25658-36-0
pyridine-2,4-dicarboxylic acid dimethyl ester

| Conditions | Yield |
|---|---|
| With thionyl chloride for 5h; Reflux; | 98% |
| With phosphorus pentachloride at 20℃; for 0.75h; | 96% |
| With thionyl chloride at 65℃; for 10h; | 90% |
-
-
499-80-9
pyridine-2,4-dicarboxylic acid

-
-
100-61-8
N-methylaniline

-
-
94870-72-1
pyridine-2,4-dicarboxylic acid bis-(N-methyl-anilide)

| Conditions | Yield |
|---|---|
| Stage #1: pyridine-2,4-dicarboxylic acid With thionyl chloride for 12h; Reflux; Stage #2: N-methylaniline With dmap; triethylamine In dichloromethane at 0 - 20℃; | 97% |

| Conditions | Yield |
|---|---|
| With tert-butylhypochlorite; chloranil at 110℃; for 30h; Inert atmosphere; | 93% |
-
-
499-80-9
pyridine-2,4-dicarboxylic acid

| Conditions | Yield |
|---|---|
| With perchloric acid at 120℃; for 72h; | 90% |

-
-
499-80-9
pyridine-2,4-dicarboxylic acid

-
-
10025-98-6
potassium tetrachloropalladate(II)

| Conditions | Yield |
|---|---|
| In water High Pressure; aq. soln. of Pd complex, dicarboxylic acid, and 1M KOH mixed; aq. soln. of metal chloride added (Pd:metal:ligand:base = 1:2:2:6); heated in a Teflon-lined autoclave at 200°C for 15 h, cooled to room temp. overa period of 8 h; ppt. filtered off, washed (H2O), dried; elem. anal.; | 89% |


-
-
499-80-9
pyridine-2,4-dicarboxylic acid

-
-
7732-18-5
water

-
-
363176-04-9, 831196-27-1
[Cu(pyridine-2,4-dicarboxylic acid(-1H))2(H2O)2]

| Conditions | Yield |
|---|---|
| In methanol; water MeOH soln. of ligand (2 equiv.) added to aq. soln. of Cu salt; filtered; ppt. washed with MeOH; dried under vac. for 1 h; elem. anal.; | 88% |


| Conditions | Yield |
|---|---|
| In water by addn. of an aq. soln. of a ligand (0.5 mmol) and hydrazine hydrate (2mmol) to the aq. soln. of metal nitrate hydrate (0.5 mmol); the ppt. was collected, washed with water, ethanol, and diethyl ether, and air-dried; elem. anal.; | 88% |

-
-
499-80-9
pyridine-2,4-dicarboxylic acid

| Conditions | Yield |
|---|---|
| In water High Pressure; a mixt. of Tb-contg. compd. (0.1 mmol), Cu-contg. compd. (0.15 mmol) anda ligand (0.3 mmol) in H2O was sealed in a Teflon-lined bomb at 160.deg ree.C for 6 d; slow cooling to room temp. by 5°C/h; crystals were recovered by filtration, washed by distd. water, and air-dried; elem. anal.; | 87% |


| Conditions | Yield |
|---|---|
| Stage #1: Creatinine; pyridine-2,4-dicarboxylic acid In water at 80℃; for 1h; Stage #2: cobalt(II) chloride hexahydrate In water at 80℃; for 1h; | 85.54% |

-
-
499-80-9
pyridine-2,4-dicarboxylic acid

-
-
7732-18-5
water

-
-
6046-93-1
copper(II) acetate monohydrate

-
-
13362-78-2
trans-1,2-bis(pyridin-4-yl)ethene

| Conditions | Yield |
|---|---|
| With Et3N In methanol; water aq. soln. of copper compd. mixed with soln. of pyridinedicarboxylic acidand Et3N (1:1:2), stirred for 15 min, methanolic soln. of bpe (2 equiv. )added; stored for several d at room temp., crystd., elem. anal.; | 85% |


| Conditions | Yield |
|---|---|
| Stage #1: pyridine-2,4-dicarboxylic acid; copper(II) nitrate trihydrate; water With triethylamine In methanol for 0.166667h; Stage #2: 2-(Aminomethyl)pyridine In methanol for 1h; pH=Ca. 7 - 8; Reflux; | 84% |

-
-
499-80-9
pyridine-2,4-dicarboxylic acid

| Conditions | Yield |
|---|---|
| In water High Pressure; a mixt. of Er-contg. compd. (0.1 mmol), Cu-contg. compd. (0.15 mmol) anda ligand (0.3 mmol) in H2O was sealed in a Teflon-lined bomb at 160.deg ree.C for 6 d; slow cooling to room temp. by 5°C/h; crystals were recovered by filtration, washed by distd. water, and air-dried; elem. anal.; | 83% |

-
-
499-80-9
pyridine-2,4-dicarboxylic acid

-
-
10025-98-6
potassium tetrachloropalladate(II)

| Conditions | Yield |
|---|---|
| In water High Pressure; aq. soln. of Pd complex, dicarboxylic acid, and 1M KOH mixed; aq. soln. of metal chloride added (Pd:metal:ligand:base = 1:2:2:6); heated in a Teflon-lined autoclave at 200°C for 15 h, cooled to room temp. overa period of 8 h; ppt. filtered off, washed (H2O), dried; elem. anal.; | 82% |


| Conditions | Yield |
|---|---|
| In sulfuric acid dissoln. on heating; ppt. filtration off, washing, drying (over CaCl2); elem. anal.; | 82% |

| Conditions | Yield |
|---|---|
| In water by addn. of an aq. soln. of a ligand and hydrazine hydrate to the aq. soln. of metal nitrate hydrate (1:1:6 molar ratio of Ni nitrate, acid, andhydrazine hydrate); the ppt. was collected, washed with water, ethanol, and diethyl ether, and air-dried; elem. anal.; | 79% |

-
-
499-80-9
pyridine-2,4-dicarboxylic acid

| Conditions | Yield |
|---|---|
| With AgNO3 In water byproducts: AgBr; to soln. Re complex in water (pH 2.2) AgNO3 was added, stirred at room temp. for 24 h, soln. was filtered, 2,4-pyridinedicarboxylic acid was added, stirred for 36 h; ppt. was filtered off and dried; elem. anal.; | 79% |
-
-
10025-99-7
potassium tetrachloroplatinate(II)

-
-
499-80-9
pyridine-2,4-dicarboxylic acid

| Conditions | Yield |
|---|---|
| In water High Pressure; aq. soln. of Pt complex, dicarboxylic acid, and 1M KOH mixed; aq. soln. of metal chloride added (Pt:metal:ligand:base = 1:2:2:7); heated in a Teflon-lined autoclave at 200°C for 15 h, cooled to room temp. overa period of 8 h; ppt. filtered off, washed (H2O), dried; elem. anal.; | 78% |


| Conditions | Yield |
|---|---|
| In water High Pressure; hydrothermal conditions; mixt. of Ni powder and 2,4-pyridinedicarboxylicacid (molar ratio 1.5:1) in H2O placed in stainless steel autoclave; he ated at 170°C under autogenous pressure for 3 d; cooled to room temp. (5°C/h); crystals isolated; elem. anal.; | 78% |

-
-
499-80-9
pyridine-2,4-dicarboxylic acid

| Conditions | Yield |
|---|---|
| With KOH In water High Pressure; 2 equiv. of the N-compd. in 1.0 M KOH and H2O were added to a CuCl2 soln., 1 equiv. of aq. MnCL2 was added to this soln., Cu:OH:ratio was 1:4, teflon-lined steel autoclave, 200 °C for 17 h; cooling to room temp. over 10 mins, crystals were filtered off, elem. anal.; | 78% |

-
-
499-80-9
pyridine-2,4-dicarboxylic acid

| Conditions | Yield |
|---|---|
| In water at 199.84℃; for 0.5h; pH=8; Time; Microwave irradiation; | 78% |

-
-
499-80-9
pyridine-2,4-dicarboxylic acid

-
-
58298-10-5
[Re2Cl4(μ-bis(diphenylphosphino)methane)2]

-
-
75-09-2
dichloromethane

-
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| In ethanol mixt. Re2Cl4(μ-dppm)2 and pyridine-2,4-dicarboxylic acid in EtOH was refluxed for 24 h; react. mixt. was filtered, residue was washed with EtOH and Et2O and dried in vacuo, recrystn. from benzene-CH2Cl2; elem. anal.; | 77% |

-
-
499-80-9
pyridine-2,4-dicarboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In dimethyl sulfoxide at 159.99℃; for 96h; Sealed tube; | 77% |
-
-
499-80-9
pyridine-2,4-dicarboxylic acid

-
-
10025-98-6
potassium tetrachloropalladate(II)

| Conditions | Yield |
|---|---|
| In water High Pressure; aq. soln. of Pd complex, dicarboxylic acid, and 1M KOH mixed; aq. soln. of metal chloride added (Pd:metal:ligand:base = 1:2:2:6); heated in a Teflon-lined autoclave at 200°C for 15 h, cooled to room temp. overa period of 8 h; ppt. filtered off, washed (H2O), dried; elem. anal.; | 75% |


| Conditions | Yield |
|---|---|
| With KOH; H2O In water A mixt. of Cu-salt, acid, KOH (pH 3) and H2O was heated at 180°C for 3 days, cooled to room temp. by air-cooling; ppt. was filtered, washed with H2O and air-dried; elem. anal.; | 75% |
2,4-Pyridinedicarboxylic acid Specification
The 2,4-Pyridinedicarboxylic acid, with the CAS registry number 499-80-9, is also known as Lutidinic acid. Its EINECS registry number is 207-892-2. This chemical's molecular formula is C7H5NO4 and molecular weight is 167.12. What's more, both its IUPAC name and systematic name are the same which is called Pyridine-2,4-dicarboxylic acid. It is used as organic synthetic reagent.
Physical properties about 2,4-Pyridinedicarboxylic acid are: (1)ACD/LogP: -0.20; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -3.84; (4)ACD/LogD (pH 7.4): -4.34; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 1; (8)ACD/KOC (pH 7.4): 1; (9)#H bond acceptors: 5; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 65.49 Å2; (13)Index of Refraction: 1.627; (14)Molar Refractivity: 38.2 cm3; (15)Molar Volume: 107.7 cm3; (16)Surface Tension: 83.5 dyne/cm; (17)Density: 1.551 g/cm3; (18)Flash Point: 301.4 °C; (19)Enthalpy of Vaporization: 90.58 kJ/mol; (20)Boiling Point: 574.8 °C at 760 mmHg; (21)Vapour Pressure: 4.71E-14 mmHg at 25 °C.
Preparation of 2,4-Pyridinedicarboxylic acid: this chemical can be prepared by 2,4-Dimethyl-pyridine. This reaction needs reagents oxygen, Cu(NO3)2, H2O at temperature of 225-230 °C.
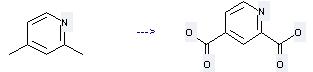
When you are dealing with this chemical, you should be very careful. This chemical is inflammation to the skin, eyes and respiratory system or other mucous membranes. Therefore, you should avoid contacting with skin and eyes. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: O=C(O)c1nccc(C(=O)O)c1
(2) InChI: InChI=1S/C7H5NO4/c9-6(10)4-1-2-8-5(3-4)7(11)12/h1-3H,(H,9,10)(H,11,12)
(3) InChIKey: MJIVRKPEXXHNJT-UHFFFAOYSA-N
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 499-81-0
- 499-83-2
- 4998-38-3
- 4998-57-6
- 499-86-5
- 4998-76-9
- 499-90-1
- 4999-79-5
- 50-00-0
- 500008-45-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View