-
Name
2-Aminopyridine
- EINECS 207-988-4
- CAS No. 504-29-0
- Article Data139
- CAS DataBase
- Density 1.107g/cm3
- Solubility Slightly soluble in water: 1-5 g/100 mL at 19°C
- Melting Point 59 °C
- Formula C5H6N2
- Boiling Point 210.6 °C at 760 mmHg
- Molecular Weight 94.116
- Flash Point 98.7 °C
- Transport Information UN 2671 6.1/PG 2
- Appearance cream to light yellow-beige crystalline powder
- Safety 26-36/37/39-45-38-28B
- Risk Codes 21-25-36/37/38-23/24/25
-
Molecular Structure
-
Hazard Symbols
 T,
T, Xi
Xi
- Synonyms alpha-Aminopyridine;1,2-Dihydro-2-iminopyridine;2-Pyridinamine;AI3-15287;Amino-2 pyridine;CCRIS 4747;HSDB 2068;NSC 431;alpha-Aminopyridine;alpha-Pyridinamine;o-Aminopyridine;2-Pyridylamine;Pyridine, 2-amino-;o-Aminopyridine;
- PSA 38.91000
- LogP 1.24500
Synthetic route

| Conditions | Yield |
|---|---|
| With [Cu2(2,7-bis(pyridin-2-yl)-l,8-naphthyridine)(OH)(CF3COO)3]; tetrabutylammomium bromide; ammonia; caesium carbonate In water at 110 - 120℃; for 16h; Sealed tube; | 100% |
| With ammonia; triethylamine In water at 20℃; for 4.25h; | 97% |
| With ammonium hydroxide at 20℃; for 7h; Catalytic behavior; | 96% |
-
-
34813-97-3
2-(formylamino)pyridine

-
-
504-29-0
2-aminopyridine

| Conditions | Yield |
|---|---|
| dodecacarbonyl-triangulo-triruthenium In acetonitrile for 2h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; toluene-4-sulfonic acid In 1,2-dichloro-ethane at 20℃; for 1h; Reagent/catalyst; Solvent; Schlenk technique; chemoselective reaction; | A n/a B 99% |

-
A
-
504-29-0
2-aminopyridine

-
B
-
1029542-72-0
N-Cyclohexyl-2-(4-fluorophenyl)-2-oxoacetamide

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; toluene-4-sulfonic acid In 1,2-dichloro-ethane at 20℃; for 1h; Schlenk technique; chemoselective reaction; | A n/a B 99% |
-
-
1218933-56-2
2‑(4‑bromophenyl)‑N‑cyclohexylimidazo[1,2‑a]pyridin‑3‑amine

-
A
-
504-29-0
2-aminopyridine

-
B
-
1029542-48-0
2-(4-bromophenyl)-N-cyclohexyl-2-oxoacetamide

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; toluene-4-sulfonic acid In 1,2-dichloro-ethane at 20℃; for 1h; Schlenk technique; chemoselective reaction; | A n/a B 98% |
-
-
39910-65-1
2-azido pyridine

-
-
504-29-0
2-aminopyridine

| Conditions | Yield |
|---|---|
| With iron(III) oxide; hydrazine hydrate In water at 120℃; for 1.5h; Inert atmosphere; | 97% |
| With D-glucose; potassium hydroxide In water at 85℃; for 0.166667h; Green chemistry; chemoselective reaction; | 97% |
| Stage #1: pyridine-2-azide With hydrazine hydrate for 0.166667h; Inert atmosphere; Stage #2: for 12h; Irradiation; chemoselective reaction; | 80% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 20℃; for 16h; Sealed tube; | A 39 mg B 97% |
-
-
601468-08-0
N‑(tert‑butyl)‑2‑(4‑chlorophenyl)imidazo[1,2‑a]pyridin‑3‑amine

-
A
-
504-29-0
2-aminopyridine

-
B
-
69770-99-6
N-(tert-butyl)-2-(4-chlorophenyl)-2-oxoacetamide

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; toluene-4-sulfonic acid In 1,2-dichloro-ethane at 20℃; for 1h; Schlenk technique; chemoselective reaction; | A n/a B 97% |
-
-
1152035-02-3
2-(3-bromophenyl)-N-cyclohexylimidazolo[1,2-a]pyridin-3-amine

-
A
-
504-29-0
2-aminopyridine

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; toluene-4-sulfonic acid In 1,2-dichloro-ethane at 20℃; for 1h; Schlenk technique; chemoselective reaction; | A n/a B 97% |

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium (0); lithium hexamethyldisilazane; CyJohnPhos In tetrahydrofuran at 65℃; for 15h; | 96% |
| With dicyclohexyl(2',4',6'-triisopropyl-5-methoxy-3,4,6-trimethyl-[1,1'-biphenyl]-2-yl)phosphine; C50H70NO4PPdS; C50H70NO4PPdS; dicyclohexyl(2',4',6'-triisopropyl-4-methoxy-3,5,6-trimethyl-[1,1'-biphenyl]-2-yl)phosphine; ammonia; sodium t-butanolate In 1,4-dioxane at 60℃; for 24h; Inert atmosphere; | 93% |
| With ammonia; zinc(II) chloride at 220℃; |

-
-
504-29-0
2-aminopyridine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In tetrahydrofuran at 20℃; | 96% |
-
-
552855-95-5
N-tert-butyl-2-(4-fluorophenyl)-1H-imidazo[1,2-a]pyridine-3-amine

-
A
-
504-29-0
2-aminopyridine

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; toluene-4-sulfonic acid In 1,2-dichloro-ethane at 20℃; for 1h; Schlenk technique; chemoselective reaction; | A n/a B 96% |
-
-
39080-52-9
3-ethoxy-3-oxo-2-(pyridine-2-yl-aminomethylene)-propanoic acid ethyl ester

-
-
504-29-0
2-aminopyridine

| Conditions | Yield |
|---|---|
| With ethylenediamine In ethanol at 20℃; for 1.3h; | 95% |
-
-
96354-74-4
N-(diphenylmethyl)pyridin-2-amine

-
A
-
504-29-0
2-aminopyridine

-
B
-
90-99-3
diphenylchloromethane

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 20℃; for 16h; Sealed tube; | A 41 mg B 95% |

-
A
-
504-29-0
2-aminopyridine

-
B
-
24914-10-1
2-(4-chlorophenyl)-N-cyclohexyl-2-oxoacetamide

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; toluene-4-sulfonic acid In 1,2-dichloro-ethane at 20℃; for 1h; Schlenk technique; chemoselective reaction; | A n/a B 95% |

-
A
-
504-29-0
2-aminopyridine

-
B
-
21010-60-6
N-(tert-butyl)-2-oxo-2-phenylacetamide

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; toluene-4-sulfonic acid In 1,2-dichloro-ethane at 20℃; for 1h; Schlenk technique; chemoselective reaction; | A n/a B 95% |

-
A
-
504-29-0
2-aminopyridine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 80℃; for 16h; Sealed tube; | A 39 mg B 94% |

-
A
-
504-29-0
2-aminopyridine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 20℃; for 16h; Sealed tube; | A 43 mg B 94% |

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; water-d2; toluene-4-sulfonic acid In 1,2-dichloro-ethane at 20℃; | A n/a B 94% |

-
A
-
504-29-0
2-aminopyridine

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; toluene-4-sulfonic acid In 1,2-dichloro-ethane at 20℃; for 1h; Schlenk technique; chemoselective reaction; | A n/a B 94% |

-
A
-
504-29-0
2-aminopyridine

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; toluene-4-sulfonic acid In 1,2-dichloro-ethane at 20℃; for 1h; Schlenk technique; chemoselective reaction; | A n/a B 93% |

-
A
-
504-29-0
2-aminopyridine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 20℃; for 16h; Sealed tube; | A 43 mg B 91% |
-
-
855140-42-0
N-cyclohexyl-2-(2-methoxyphenyl)imidazo[1,2-a]pyridin-3-amine

-
A
-
504-29-0
2-aminopyridine

-
B
-
108842-43-9
<2-Methoxy-phenyl>-glyoxylsaeure-cyclohexylamid

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; toluene-4-sulfonic acid In 1,2-dichloro-ethane at 20℃; for 1h; Schlenk technique; chemoselective reaction; | A n/a B 91% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; potassium phosphate; 1-(5,6,7,8-tetrahydroquinolin-8-yl)-2-methylpropan-1-one; copper(I) bromide In dimethyl sulfoxide at 25℃; for 24h; Inert atmosphere; Sealed tube; | 89% |
| With copper(I) oxide; ammonium hydroxide; potassium carbonate at 140℃; for 16h; Inert atmosphere; | 82% |
| With acetamidine hydrochloride; caesium carbonate In N,N-dimethyl-formamide at 130℃; for 20h; Inert atmosphere; Green chemistry; | 81% |
| With ammonium hydroxide; copper(l) iodide; N,N'-bis(3,5-dimethoxyphenyl)cyclopentane-1,1-dicarboxamide; caesium carbonate In dimethyl sulfoxide at 20℃; for 24h; Inert atmosphere; Sealed tube; | 59% |
| Multi-step reaction with 2 steps 1: xantphos; Cs2CO3 / Pd(OAc)2 / dioxane / 24 h / 100 °C 2: trifluoroacetic acid / CH2Cl2 / 20 °C View Scheme |

-
A
-
504-29-0
2-aminopyridine

-
B
-
1029542-43-5
N-cyclohexyl-2-oxo-2-(o-tolyl)acetamide

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; toluene-4-sulfonic acid In 1,2-dichloro-ethane at 20℃; for 1h; Schlenk technique; chemoselective reaction; | A n/a B 89% |
2-AMINOPYRIDINE Chemical Properties
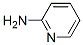
Formula Weight: 94.11
mp of 2-AMINOPYRIDINE (504-29-0): 59 °C
Density of 2-AMINOPYRIDINE (504-29-0): 1.107 g/cm3
Boiling Point: 210.6 °C at 760 mmHg
Flash Point: 98.7 °C
Vapour Pressure: 0.191 mmHg at 25°C
Index of Refraction: 1.587
Water Solubility : Slightly soluble. 1-5 g/100 mL at 19 oC
Appearance of 2-AMINOPYRIDINE (504-29-0): White crystalline solid, faint, sweet, somewhat balsamic and coumarin-like odour
2-AMINOPYRIDINE Uses
2-AMINOPYRIDINE Toxicity Data With Reference
| 1. | ihl-hmn TCLo:5 ppm/5H:CNS | IMSUAI Industrial Medicine and Surgery. 19 (1950),317. | ||
| 2. | orl-rat LD50:200 mg/kg | 85JCAE Prehled Prumyslove Toxikologie; Organicke Latky Marhold, J.,Prague, Czechoslovakia.: Avicenum,1986,838. | ||
| 3. | ipr-mus LD50:35 mg/kg | JMCMAR Journal of Medicinal Chemistry. 8 (1965),296. | ||
| 4. | scu-mus LD50:70 mg/kg | AEPPAE Naunyn-Schmiedeberg’s Archiv fuer Experimentelle Pathologie und Pharmakologie. 226 (1955),163. | ||
| 5. | ivn-mus LD50:23 mg/kg | APFRAD Annales Pharmaceutiques Francaises. 26 (1968),345. | ||
| 6. | orl-qal LD50:133 mg/kg | AECTCV Archives of Environmental Contamination and Toxicology. 12 (1983),355. | ||
| 7. | orl-bwd LD50:31,600 µg/kg | AECTCV Archives of Environmental Contamination and Toxicology. 12 (1983),355. |
2-AMINOPYRIDINE Consensus Reports
2-AMINOPYRIDINE Safety Profile
Risk Statements : 21-25-36/37/38-23/24/25 (Harmful in contact with skin; Toxic if swallowed; Irritating to eyes, respiratory system and skin; Toxic by inhalation, in contact with skin and if swallowed)
Safety Statements : 26-36/37/39-45-38-28B (In case of contact with eyes, rinse immediately with plenty of water and seek medical advice; Wear suitable protective clothing, gloves and eye/face protection; In case of accident or if you feel unwell, seek medical advice immediately; In case of insufficient ventilation, wear suitable respiratory equipment; After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer) )
RIDADR : UN 2671 6.1/PG 2
WGK Germany : 3
RTECS : US1575000
F : 8-21 (Photosensitive; Sensitive to humidity)
Hazard Note : Toxic/Irritant
HazardClass : 6.1
PackingGroup : II
HS Code : 29333999
Poison by ingestion, inhalation, subcutaneous, intravenous, and intraperitoneal routes. Toxic effects resemble strychnine poisoning. Human systemic effects by inhalation: somnolence, convulsions, and antipsychotic effects. Human central nervous system effects by inhalation. When heated to decomposition it emits highly toxic fumes of NOx.
2-AMINOPYRIDINE Standards and Recommendations
ACGIH TLV: TWA 0.5 ppm
DFG MAK: 0.5 ppm (2 mg/m3)
DOT Classification: 6.1; Label: Poison
2-AMINOPYRIDINE Analytical Methods
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View