-
Name
2-AMINO-1-BUTANOL
- EINECS 202-488-2
- CAS No. 96-20-8
- Article Data39
- CAS DataBase
- Density 0.944 g/mL at 20 °C(lit.)
- Solubility Completely miscible in water
- Melting Point -2 °C(lit.)
- Formula C4H11NO
- Boiling Point 177.2 °C at 760 mmHg
- Molecular Weight 89.1374
- Flash Point 82.2 °C
- Transport Information UN 2735 8/PG 3
- Appearance clear liquid
- Safety 26-36/37/39-45
- Risk Codes 34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms (RS)-2-Amino-1-butanol;1-(Hydroxymethyl)propylamine;1-Hydroxy-2-aminobutane;1-Hydroxy-2-butylamine;1-Hydroxy-sec-butylamine;1-Hydroxybutan-2-amine;2-Amino-1-butanol;2-Amino-1-hydroxybutane;2-Aminobutyl alcohol;DL-2-Amino-1-butanol;DL-2-Aminobutanol;DL-a-Aminobutanol;NSC 1068;1-Butanol, 2-amino-;
- PSA 46.25000
- LogP 0.41630
2-Aminobutanol Consensus Reports
2-Aminobutanol Standards and Recommendations
SPECIFIC GRAVITY: 0.94 - 0.95
WATER: 0.5% max
2-Aminobutanol Specification
The 2-Aminobutan-1-ol, with the CAS registry number 96-20-8, is also known as Butanol-2-amine. It belongs to the product categories of Amino Alcohols; Organic Building Blocks; Oxygen Compounds. Its EINECS registry number is 202-488-2. This chemical's molecular formula is C4H11NO and molecular weight is 89.14. Its IUPAC name is called 2-aminobutan-1-ol. What's more, this chemical can be used for the preparation of emulsifying agent, surfactant and vulcanization acclerant. It also can be used in organic synthesis and used as absorption agent of acidic gas.
Physical properties of 2-Aminobutan-1-ol: (1)ACD/LogP: -0.43; (2)ACD/LogD (pH 5.5): -3.52; (3)ACD/LogD (pH 7.4): -2.98; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 1; (7)ACD/KOC (pH 7.4): 1; (8)#H bond acceptors: 2; (9)#H bond donors: 3; (10)#Freely Rotating Bonds: 4; (11)Index of Refraction: 1.445; (12)Molar Refractivity: 25.6 cm3; (13)Molar Volume: 96.1 cm3; (14)Surface Tension: 35.3 dyne/cm; (15)Density: 0.927 g/cm3; (16)Flash Point: 82.2 °C; (17)Enthalpy of Vaporization: 48.14 kJ/mol; (18)Boiling Point: 177.2 °C at 760 mmHg; (19)Vapour Pressure: 0.319 mmHg at 25°C.
Preparation: this chemical can be prepared by 1-butene, acetonitrile and chlorine via Chlorinated addition reaction. This reaction will need Hydrolysis, alcoholize and alkalify. Finally, it will gain 2-Aminobutan-1-ol.
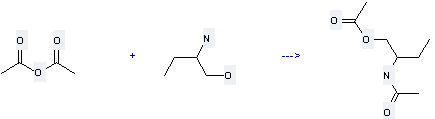
Uses of 2-Aminobutan-1-ol: it can be used to produce 1-acetoxy-2-acetylamino-butane with acetic acid anhydride at temperature of 80 °C. The yield is about 91%.
When you are using this chemical, please be cautious about it as the following:
This chemical may destroy living tissue on contact and may cause burns. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. Whenever you will contact it, please wear suitable protective clothing, gloves and eye/face protection. In case of accident or if you feel unwell seek medical advice immediately (show the label where possible).
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: CCC(CO)N
(2)InChI: InChI=1S/C4H11NO/c1-2-4(5)3-6/h4,6H,2-3,5H2,1H3
(3)InChIKey: JCBPETKZIGVZRE-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intravenous | 316mg/kg (316mg/kg) | U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. Vol. NX#00036, | |
| mouse | LD50 | oral | 2300mg/kg (2300mg/kg) | BEHAVIORAL: TETANY | "Novye Dannye Po Toksikologii Redkikh Metalov Ikh Soedinenii," New Data on the Toxicology of Rare Metals and Their Compounds, Izrael'son, Z.I., ed., Moscow, Izdatel'stvo "Meditsina," 196Vol. -, Pg. -, 1967. |
| mouse | LDLo | intraperitoneal | 250mg/kg (250mg/kg) | "Summary Tables of Biological Tests," National Research Council Chemical-Biological Coordination Center. Vol. 5, Pg. 338, 1953. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View