-
Name
2-Aminopyrimidine-5-carboxylic acid
- EINECS
- CAS No. 3167-50-8
- Article Data11
- CAS DataBase
- Density 1.534 g/cm3
- Solubility
- Melting Point >300 °C
- Formula C5H5N3O2
- Boiling Point 474.584 °C at 760 mmHg
- Molecular Weight 139.114
- Flash Point 240.82 °C
- Transport Information
- Appearance
- Safety 36/37-24/25-22
- Risk Codes 20/21/22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2-Amino-5-carboxypyrimidine;2-Amino-5-pyrimidinecarboxylic acid;
- PSA 89.10000
- LogP 0.33820
Synthetic route
-
-
308348-93-8
2-amino-pyrimidine-5-carboxylic acid methyl ester

-
-
3167-50-8
2-amino-pyrimidine-5-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2-amino-pyrimidine-5-carboxylic acid methyl ester With methanol; lithium hydroxide; water at 60℃; Stage #2: With hydrogenchloride; water In methanol pH=4; | 90% |
| Stage #1: 2-amino-pyrimidine-5-carboxylic acid methyl ester With water; lithium hydroxide In methanol at 60℃; Stage #2: With hydrogenchloride In water pH=4; | 90% |
| Stage #1: 2-amino-pyrimidine-5-carboxylic acid methyl ester With water; lithium hydroxide In methanol at 60℃; Stage #2: With hydrogenchloride In water pH=4; | 90% |
| With lithium hydroxide In methanol; water at 60℃; | 90% |
| With water; lithium hydroxide In tetrahydrofuran; methanol at 20℃; for 3h; |
-
-
7424-91-1
methyl 3,3-dimethoxypropionate

-
-
107-31-3
Methyl formate

-
-
3167-50-8
2-amino-pyrimidine-5-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: methyl 3,3-dimethoxypropionate With sodium methylate In 1,4-dioxane; methanol Reflux; Large scale; Stage #2: Methyl formate In 1,4-dioxane; methanol at 20℃; Large scale; Stage #3: guanidine hydrochloride salt Large scale; Further stages; | 65% |

-
-
7424-91-1
methyl 3,3-dimethoxypropionate

-
-
107-31-3
Methyl formate

-
-
50-01-1
guanidine hydrochloride

-
-
3167-50-8
2-amino-pyrimidine-5-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: methyl 3,3-dimethoxypropionate With sodium methylate In 1,4-dioxane; methanol Reflux; Large scale; Stage #2: Methyl formate Large scale; Stage #3: guanidine hydrochloride Large scale; Further stages; | 65% |

-
-
57401-76-0
2-aminopyrimidine-5-carboxylic acid ethyl ester

-
-
3167-50-8
2-amino-pyrimidine-5-carboxylic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol for 5h; Heating; | 60% |
-
-
89793-12-4
ethyl 2-chloropyrimidine-5-carboxylate

-
-
3167-50-8
2-amino-pyrimidine-5-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NH3 View Scheme |
-
-
151323-66-9
ethyl 2-(ethylthio)pyrimidine-5-carboxylate

-
-
3167-50-8
2-amino-pyrimidine-5-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: H2O; chlorine 2: NH3 View Scheme |
-
-
2223-96-3
4-chloro-2-ethylsulfanyl-pyrimidine-5-carboxylic acid ethyl ester

-
-
3167-50-8
2-amino-pyrimidine-5-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: aqueous ethanol; zinc-powder 2: H2O; chlorine 3: NH3 View Scheme |
-
-
3167-50-8
2-amino-pyrimidine-5-carboxylic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride; platinum(IV) oxide; hydrogen In water for 48h; | 96% |
| With hydrogenchloride; hydrogen; palladium on activated charcoal under 1499.7 Torr; for 4h; Ambient temperature; | 95% |
-
-
3167-50-8
2-amino-pyrimidine-5-carboxylic acid

-
-
1032570-74-3
7-methoxy-8-[3-(morpholin-4-yl)propoxy]-2,3-dihydroimidazo[1,2-c]quinazolin-5-amine

-
-
1032568-63-0
2-amino-N-{7-methoxy-8-[3-(4 morpholinyl)propoxy]-2,3-dihydroimidazo[1,2-c]quinazolin-5-yl}-5- pyrimidinecarboxamide

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 17h; Large scale; | 96% |
| With dmap; C9H19N3*ClH In ethanol; N,N-dimethyl-formamide at 20℃; for 15h; | 96% |
| With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; | 40% |
| With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; | 40% |
-
-
3167-50-8
2-amino-pyrimidine-5-carboxylic acid


| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 0 - 20℃; for 2h; | 79% |

| Conditions | Yield |
|---|---|
| for 3h; Heating; | 74% |
-
-
3167-50-8
2-amino-pyrimidine-5-carboxylic acid


| Conditions | Yield |
|---|---|
| With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 24h; | 68% |
-
-
3167-50-8
2-amino-pyrimidine-5-carboxylic acid

-
-
1428929-08-1, 1428954-51-1
4-[[3-[[5-(trifluoromethyl)-2-pyridyl]oxy]phenyl]methylene]-cyclohexanamine

-
-
1428926-93-5, 1428947-75-4, 1428949-41-0
2-amino-N-[4-[[3-[[5-(trifluoromethyl)-2-pyridyl]oxy]phenyl] methylene]cyclohexyl]pyrimidine-5-carboxamide

| Conditions | Yield |
|---|---|
| With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 18h; | 62% |
-
-
3167-50-8
2-amino-pyrimidine-5-carboxylic acid

| Conditions | Yield |
|---|---|
| With DBN; (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate In acetonitrile at 20℃; for 12h; | 61.1% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-amino-pyrimidine-5-carboxylic acid With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 0.166667h; Stage #2: 2-methyltryptamine In N,N-dimethyl-formamide at 20℃; for 24h; | 59% |
-
-
3167-50-8
2-amino-pyrimidine-5-carboxylic acid

-
-
57260-71-6
1-t-Butoxycarbonylpiperazine

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In N,N-dimethyl-formamide at 20℃; for 12h; | 56.6% |
-
-
3167-50-8
2-amino-pyrimidine-5-carboxylic acid

| Conditions | Yield |
|---|---|
| With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; | 54% |
-
-
3167-50-8
2-amino-pyrimidine-5-carboxylic acid

| Conditions | Yield |
|---|---|
| With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 48h; | 40% |
-
-
3167-50-8
2-amino-pyrimidine-5-carboxylic acid

-
-
1202649-62-4
3-amino-4-methyl-N-(4-((4-methylpiperazin-1-yl) methyl)-3-(trifluoromethyl)phenyl)benzamide

-
-
1581701-25-8
2-amino-N-(2-methyl-5-((4-((4-methylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)carbamoyl)phenyl)pyrimidine-5-carboxamide

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 20℃; for 12h; | 38% |
-
-
3167-50-8
2-amino-pyrimidine-5-carboxylic acid

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 20℃; | 35% |
-
-
3167-50-8
2-amino-pyrimidine-5-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 95 percent / H2, aq. HCl / 10percent Pd/C / 4 h / 1499.7 Torr / Ambient temperature 2: 0.53 g / oxalyl chloride / benzene / 2.5 h / Heating View Scheme |
-
-
3167-50-8
2-amino-pyrimidine-5-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 95 percent / H2, aq. HCl / 10percent Pd/C / 4 h / 1499.7 Torr / Ambient temperature 2: 81.2 percent / SOCl2 / Heating View Scheme |
-
-
3167-50-8
2-amino-pyrimidine-5-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 95 percent / H2, aq. HCl / 10percent Pd/C / 4 h / 1499.7 Torr / Ambient temperature 2: 82.1 percent / SOCl2 / Heating View Scheme |
-
-
3167-50-8
2-amino-pyrimidine-5-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 95 percent / H2, aq. HCl / 10percent Pd/C / 4 h / 1499.7 Torr / Ambient temperature 2: 34.2 percent / SOCl2 / Heating View Scheme |
-
-
3167-50-8
2-amino-pyrimidine-5-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 95 percent / H2, aq. HCl / 10percent Pd/C / 4 h / 1499.7 Torr / Ambient temperature 2: 75.2 percent / SOCl2 / Heating View Scheme |
-
-
3167-50-8
2-amino-pyrimidine-5-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 95 percent / H2, aq. HCl / 10percent Pd/C / 4 h / 1499.7 Torr / Ambient temperature 2: 0.53 g / oxalyl chloride / benzene / 2.5 h / Heating 3: 53.2 percent / Ambient temperature View Scheme |
-
-
3167-50-8
2-amino-pyrimidine-5-carboxylic acid

-
-
69034-08-8
5-(trifluoromethyl)pyrimidin-2-amine

| Conditions | Yield |
|---|---|
| With HF; SF4 |
-
-
3167-50-8
2-amino-pyrimidine-5-carboxylic acid

-
-
544-00-3
3-methyl-N-(3-methylbutyl)-1-butanamine

-
-
1187663-51-9
C15H26N4O

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 50℃; |

| Conditions | Yield |
|---|---|
| With scandium tris(trifluoromethanesulfonate) In methanol; dichloromethane at 20℃; for 1h; |
2-Aminopyrimidine-5-carboxylic acid Chemical Properties
Molecular Structure of 5-Pyrimidinecarboxylicacid, 2-amino- (CAS No.3167-50-8):
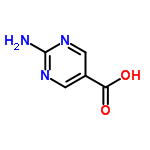
Molecular Formula: C5H5N3O2
Molecular Weight: 139.1121
IUPAC Name: 2-Aminopyrimidine-5-carboxylic acid
CAS No: 3167-50-8
H bond acceptors: 5
H bond donors: 3
Freely Rotating Bonds: 1
Polar Surface Area: 89.1 Å2
Index of Refraction: 1.663
Molar Refractivity: 33.602 cm3
Molar Volume: 90.691 cm3
Surface Tension: 95.532 dyne/cm
Density: 1.534 g/cm3
Flash Point: 240.82 °C
Enthalpy of Vaporization: 77.738 kJ/mol
Boiling Point: 474.584 °C at 760 mmHg
Vapour Pressure: 0 mmHg at 25°C
Product Categories: Aminoacid;Pyrimidine;Pharmacetical;Carboxylic Acids;Pyrazines, Pyrimidines Pyridazines;API intermediates;Carboxylic Acids;Pyrazines, Pyrimidines Pyridazines
InChI: InChI=1/C5H5N3O2/c6-5-7-1-3(2-8-5)4(9)10/h1-2H,(H,9,10)(H2,6,7,8)
InChIKey: CBRLWSXYXSFYSP-UHFFFAOYAM
Std. InChI: InChI=1S/C5H5N3O2/c6-5-7-1-3(2-8-5)4(9)10/h1-2H,(H,9,10)(H2,6,7,8)
Std. InChIKey: CBRLWSXYXSFYSP-UHFFFAOYSA-N
2-Aminopyrimidine-5-carboxylic acid Safety Profile
Safety Information of 5-Pyrimidinecarboxylicacid, 2-amino- (CAS No.3167-50-8):
Hazard Codes:  Xi
Xi
2-Aminopyrimidine-5-carboxylic acid Specification
5-Pyrimidinecarboxylicacid, 2-amino- (CAS No.3167-50-8), it also can be called 2-Aminopyrimidine-5-carboxylic acid .
Related Products
- 2-Aminopyrimidine-5-carboxylic acid
- 3167-63-3
- 31676-54-7
- 31676-55-8
- 31677-93-7
- 316800-46-1
- 316800-47-2
- 316800-48-3
- 31680-07-6
- 31680-08-7
- 316-81-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View