-
Name
2-Bromo-4'-Benzyloxy-3'-nitroacetophenone
- EINECS 610-115-3
- CAS No. 43229-01-2
- Article Data16
- CAS DataBase
- Density 1.518 g/cm3
- Solubility
- Melting Point 135-137 °C(Solv: ethanol (64-17-5))
- Formula C15H12BrNO4
- Boiling Point 465.239 °C at 760 mmHg
- Molecular Weight 350.169
- Flash Point 235.168 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 2-Bromo-1-(3-nitro-4-benzyloxyphenyl)ethanone;Ethanone,2-bromo-1-[3-nitro-4-(phenylmethoxy)phenyl]-;4'-(Benzyloxy)-2-bromo-3'-nitroacetophenone;
- PSA 72.12000
- LogP 4.27460
Synthetic route
-
-
14347-05-8
4-benzyloxy-3-nitroacetophenone

-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

| Conditions | Yield |
|---|---|
| With N,N,N-trimethylanilinium bromide In dichloromethane at 300℃; for 4h; | 98% |
| With phenyltrimethylammonium tribromide In methanol; dichloromethane at 20℃; | 97% |
| With bromine; acetic acid at 20℃; | 85% |
-
-
6322-56-1
4'-hydroxy-3'-nitroacetophenone

-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: TBAI, K2CO3 / acetone 2: Br2 / CHCl3 View Scheme | |
| Multi-step reaction with 2 steps 1.1: N-ethyl-N,N-diisopropylamine / acetonitrile / 20 °C 1.2: 3 h / 20 °C / Reflux 2.1: tetra-N-butylammonium tribromide / tetrahydrofuran; methanol / 4 h View Scheme | |
| Multi-step reaction with 2 steps 1.1: potassium carbonate / water; acetone / 20 °C 1.2: 17 h / Reflux 2.1: bromine / acetic acid / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: potassium carbonate / acetone; water / 60 °C 2: bromine / acetic acid / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: potassium carbonate; sodium iodide / acetone / 66 h / Heating / reflux 2: phenyltrimethylammonium tribromide / tetrahydrofuran / 12 h / 20 °C View Scheme |
-
-
99-93-4
4-Hydroxyacetophenone

-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: HNO3 / acetic acid / 0 °C 2: TBAI, K2CO3 / acetone 3: Br2 / CHCl3 View Scheme | |
| Multi-step reaction with 3 steps 1: HNO3 / acetic acid / -0.1 °C 2: TBAI, K2CO3 / acetone 3: Br2 / CHCl3 View Scheme |
-
-
100-39-0
benzyl bromide

-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: N-ethyl-N,N-diisopropylamine / acetonitrile / 20 °C 1.2: 3 h / 20 °C / Reflux 2.1: tetra-N-butylammonium tribromide / tetrahydrofuran; methanol / 4 h View Scheme | |
| Multi-step reaction with 2 steps 1: potassium carbonate; sodium iodide / acetone / 66 h / Heating / reflux 2: phenyltrimethylammonium tribromide / tetrahydrofuran / 12 h / 20 °C View Scheme |
-
-
100-44-7
benzyl chloride

-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: potassium carbonate / water; acetone / 20 °C 1.2: 17 h / Reflux 2.1: bromine / acetic acid / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: potassium carbonate / acetone; water / 60 °C 2: bromine / acetic acid / 20 °C View Scheme |
-
-
6322-56-1
4'-hydroxy-3'-nitroacetophenone

-
-
100-44-7
benzyl chloride

-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium carbonate; N-benzyl-N,N,N-triethylammonium chloride / N,N-dimethyl-formamide / 4 h / 115 - 120 °C 2: 2,2'-azobis(isobutyronitrile); bromine / 4 h / 38 - 42 °C View Scheme |
-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

-
-
188690-82-6
(R)-2-bromo-1-(4-benzyloxy-3-nitrophenyl)ethanol

| Conditions | Yield |
|---|---|
| With dimethylsulfide borane complex; (3aS-cis)-(-)-3,3a,8,8a-tetrahydro-2H-indeno[1,2-d]oxazole-2-isopropylborane In tetrahydrofuran at -5 - 5℃; for 0.5h; Solvent; Temperature; | 99% |
| With oxazaborolidine from cis (1R,2S)-aminoindanol and trimethylboroxine; borane In tetrahydrofuran at -15℃; | 98% |
| With borane N,N-diethylaniline complex; (1R,2S)-1-Amino-2-indanol In tetrahydrofuran at 3 - 5℃; for 3.5h; optical yield given as %ee; | 95% |
-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

-
-
193761-53-4
(S)-1-(4-(benzyloxy)-3-nitrophenyl)-2-bromoethanol

| Conditions | Yield |
|---|---|
| With oxazaborolidine from cis (1S,2R)-aminoindanol and trimethylboroxine; borane In tetrahydrofuran at -15℃; | 98% |
| With borane N,N-diethylaniline complex; (1S,2R)-1-amino-2-indanol In tetrahydrofuran at 0 - 5℃; for 2h; | 94% |
| With borane N,N-diethylaniline complex; (1S,2R)-1-amino-2-indanol In tetrahydrofuran at 0 - 5℃; for 2h; | 94% |
-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

-
-
299964-35-5
(+/-)-2-bromo-1-(4-benzyloxy-3-nitrophenyl)ethanol

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In tetrahydrofuran at 0 - 20℃; | 91% |
| With borane-THF In tetrahydrofuran at -20℃; Reduction; | 87% |
| With sodium tetrahydroborate In methanol | |
| With sodium tetrahydroborate In tetrahydrofuran |
-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

-
-
653572-66-8
3,4-bis(3,4-dimethoxyphenyl)-1H-pyrrole-2,5-dione

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; | 74.1% |
-
-
57294-38-9
N-(tert-butoxycarbonyl)-4-aminobutanoic acid

-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

-
-
1292299-09-2
2-(4-(benzyloxy)-3-nitrophenyl)-2-oxoethyl-4-(tert-butoxycarbonylamino)butanoate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 20℃; | 67% |
-
-
695-34-1
2-Amino-4-methylpyridine

-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

-
-
141244-34-0
2-(4-benzyloxy-3-nitrophenyl)-7-methylimidazo<1,2-a>pyridine

| Conditions | Yield |
|---|---|
| In acetonitrile for 7h; Heating; | 65% |
-
-
5521-58-4
5-methylpyrazin-2-amine

-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

-
-
1254709-33-5
2-(4-(benzyloxy)-3-nitrophenyl)-6-methylimidazo[1,2-a]pyrazine

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In ethanol Reflux; | 45% |
-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

-
A
-
188730-96-3
(S)-4-benzyloxy-3-nitrostyrene oxide

-
B
-
193761-53-4
(S)-1-(4-(benzyloxy)-3-nitrophenyl)-2-bromoethanol

-
C
-
188690-82-6
(R)-2-bromo-1-(4-benzyloxy-3-nitrophenyl)ethanol

| Conditions | Yield |
|---|---|
| With sodium formate; Triton X-100; (R,R)-TsDPEN; dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer In water at 28℃; for 6h; Title compound not separated from byproducts; | A 7% B n/a C n/a |

-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

-
-
51582-41-3
2-(3-benzyloxy-4-nitrophenyl)oxirane

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium tetrahydroborate 1.) MeOH; Multistep reaction; | |
| With sodium tetrahydroborate In methanol at 20℃; for 3h; |
-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

-
-
103-49-1
dibenzylamine

-
-
173283-38-0
ω-(dibenzylamino)-4-benzyloxy-3-nitroacetophenone

| Conditions | Yield |
|---|---|
| In benzene for 72h; Ambient temperature; |
-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

-
A
-
193761-53-4
(S)-1-(4-(benzyloxy)-3-nitrophenyl)-2-bromoethanol

-
B
-
188690-82-6
(R)-2-bromo-1-(4-benzyloxy-3-nitrophenyl)ethanol

| Conditions | Yield |
|---|---|
| With boron trifluoride-tetrahydrofuran complex; (-)-(1R,2S)-1-amino-2-hydroxy-1,2,3,4-tetrahydronaphthalene In tetrahydrofuran at 0℃; for 2h; Product distribution; other reag., other temp.; | |
| With borane N,N-diethylaniline complex; (S)-Corey-Bakshi-Shibata oxazaborolidine In 1,2-dimethoxyethane; dichloromethane; water at 25 - 30℃; for 9h; Corey-Itsuno reduction; Inert atmosphere; optical yield given as %ee; | |
| With borane N,N-diethylaniline complex; (1R,2S)-1-Amino-2-indanol In tetrahydrofuran at 25℃; for 1h; Concentration; enantioselective reaction; | A n/a B n/a |
| With dimethylsulfide borane complex; C9H10BNO In tetrahydrofuran at 25℃; for 2h; Temperature; Inert atmosphere; Cooling with ice; enantioselective reaction; | A n/a B n/a |
-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

-
-
876753-52-5
2-benzyloxy-5-vinylaniline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: NaBH4 / methanol / 3 h / 20 °C 2: HOAc / methanol 3: 10.6 g / triphenylphosphine; imidazole; iodine / toluene / 3 h / Heating 4: 79.5 percent / Fe; HOAc / methanol / 2 h / Heating View Scheme |
-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

-
-
515152-69-9
2-benzyloxy-5-vinylnitrobenzene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: NaBH4 / methanol / 3 h / 20 °C 2: HOAc / methanol 3: 10.6 g / triphenylphosphine; imidazole; iodine / toluene / 3 h / Heating View Scheme |
-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: NaBH4 / methanol / 3 h / 20 °C 2: HOAc / methanol 3: 10.6 g / triphenylphosphine; imidazole; iodine / toluene / 3 h / Heating 4: 79.5 percent / Fe; HOAc / methanol / 2 h / Heating 5: acetic anhydride / CH2Cl2 / 1.5 h / 0 °C 6: 1.15 g / m-CPBA; NaHCO3 / CH2Cl2; H2O / 3 h / 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1: sodium tetrahydroborate / tetrahydrofuran 2: platinum on carbon; dimethylsulfide; hydrogen 3: acetic anhydride 4: potassium carbonate / tetrahydrofuran; methanol View Scheme | |
| Multi-step reaction with 4 steps 1: sodium tetrahydroborate / tetrahydrofuran / 0 - 20 °C 2: hydrogen; platinum on carbon; dimethylsulfide 3: acetic anhydride / 0 - 20 °C 4: potassium carbonate / methanol; tetrahydrofuran View Scheme |
-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

-
-
876753-53-6
N-(2-benzyloxy-5-vinylphenyl)formamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: NaBH4 / methanol / 3 h / 20 °C 2: HOAc / methanol 3: 10.6 g / triphenylphosphine; imidazole; iodine / toluene / 3 h / Heating 4: 79.5 percent / Fe; HOAc / methanol / 2 h / Heating 5: acetic anhydride / CH2Cl2 / 1.5 h / 0 °C View Scheme |
-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

-
-
876753-51-4
(4-benzyloxy-3-nitrophenyl)ethane-1,2-diol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NaBH4 / methanol / 3 h / 20 °C 2: HOAc / methanol View Scheme |
-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

-
-
188730-94-1
(R)-1-(4-benzyloxy-3-nitrophenyl)oxirane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 87 percent / BH3*THF / tetrahydrofuran / -20 °C 2: lipase PS Celite-immobilized / various solvent(s) / 72 h / 37 °C 3: K2CO3 / methanol / 2.5 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: BH3*THF / tetrahydrofuran / -20 °C 1.2: 48 percent / lipase PS; vinyl acetate / various solvent(s) / 72 h / 37 °C 2.1: K2CO3 / methanol / 2.5 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 98 percent / oxazaborolidine from cis (1R,2S)-aminoindanol and trimethylboroxine, BH3 / tetrahydrofuran / -15 °C 2: 98 percent / aqueous sodium hydroxide / methanol / 0.5 h View Scheme | |
| Multi-step reaction with 2 steps 1: (-)-diisopinocamphenylborane chloride / dichloromethane / 20 - 30 °C / Inert atmosphere 2: potassium carbonate / tetrahydrofuran; methanol / 20 - 30 °C View Scheme |
-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

-
-
188730-96-3
(S)-4-benzyloxy-3-nitrostyrene oxide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 87 percent / BH3*THF / tetrahydrofuran / -20 °C 2: lipase PS Celite-immobilized / various solvent(s) / 72 h / 37 °C 3: K2CO3 / methanol / 2.5 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: BH3*THF / tetrahydrofuran / -20 °C 1.2: 48 percent / lipase PS; vinyl acetate / various solvent(s) / 72 h / 37 °C 2.1: K2CO3 / methanol / 2.5 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 98 percent / oxazaborolidine from cis (1S,2R)-aminoindanol and trimethylboroxine, BH3 / tetrahydrofuran / -15 °C 2: 98 percent / aqueous sodium hydroxide / methanol / 0.5 h View Scheme |
-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

-
-
73573-87-2
(R,R)-formoterol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 87 percent / BH3*THF / tetrahydrofuran / -20 °C 2: lipase PS Celite-immobilized / various solvent(s) / 72 h / 37 °C 3: K2CO3 / methanol / 2.5 h / 20 °C 4: dimethylsulfoxide / 87 h / 80 °C 5: neutral Al2O3 (activity III) 6: 67 percent / Fe turnings; 1M aq. HCl / methanol / 0.75 h / Heating 7: 69 percent / pyridine / 6.5 h / 60 °C 8: H2 / 10 percentPd/C / ethanol / 20 °C View Scheme | |
| Multi-step reaction with 7 steps 1.1: BH3*THF / tetrahydrofuran / -20 °C 1.2: 48 percent / lipase PS; vinyl acetate / various solvent(s) / 72 h / 37 °C 2.1: K2CO3 / methanol / 2.5 h / 20 °C 3.1: dimethylsulfoxide / 87 h / 80 °C 4.1: neutral Al2O3 (activity III) 5.1: 67 percent / Fe turnings; 1M aq. HCl / methanol / 0.75 h / Heating 6.1: 69 percent / pyridine / 6.5 h / 60 °C 7.1: H2 / 10 percentPd/C / ethanol / 20 °C View Scheme | |
| Multi-step reaction with 6 steps 1: 98 percent / oxazaborolidine from cis (1R,2S)-aminoindanol and trimethylboroxine, BH3 / tetrahydrofuran / -15 °C 2: 98 percent / aqueous sodium hydroxide / methanol / 0.5 h 3: 90 °C 4: H2 / PtO2 6: H2 / Pd-C / ethanol View Scheme |
-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 87 percent / BH3*THF / tetrahydrofuran / -20 °C 2: lipase PS Celite-immobilized / various solvent(s) / 72 h / 37 °C View Scheme |
-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

-
-
299964-37-7
Acetic acid (S)-1-(4-benzyloxy-3-nitro-phenyl)-2-bromo-ethyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 87 percent / BH3*THF / tetrahydrofuran / -20 °C 2: lipase PS Celite-immobilized / various solvent(s) / 72 h / 37 °C View Scheme |
-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

-
-
299964-43-5
(R)-1-(3-Amino-4-benzyloxy-phenyl)-2-[(R)-2-(4-methoxy-phenyl)-1-methyl-ethylamino]-ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 87 percent / BH3*THF / tetrahydrofuran / -20 °C 2: lipase PS Celite-immobilized / various solvent(s) / 72 h / 37 °C 3: K2CO3 / methanol / 2.5 h / 20 °C 4: dimethylsulfoxide / 87 h / 80 °C 5: neutral Al2O3 (activity III) 6: 67 percent / Fe turnings; 1M aq. HCl / methanol / 0.75 h / Heating View Scheme | |
| Multi-step reaction with 5 steps 1.1: BH3*THF / tetrahydrofuran / -20 °C 1.2: 48 percent / lipase PS; vinyl acetate / various solvent(s) / 72 h / 37 °C 2.1: K2CO3 / methanol / 2.5 h / 20 °C 3.1: dimethylsulfoxide / 87 h / 80 °C 4.1: neutral Al2O3 (activity III) 5.1: 67 percent / Fe turnings; 1M aq. HCl / methanol / 0.75 h / Heating View Scheme |
-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

-
-
245759-61-9
(R)-1-(4-Benzyloxy-3-nitro-phenyl)-2-[(R)-2-(4-methoxy-phenyl)-1-methyl-ethylamino]-ethanol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 87 percent / BH3*THF / tetrahydrofuran / -20 °C 2: lipase PS Celite-immobilized / various solvent(s) / 72 h / 37 °C 3: K2CO3 / methanol / 2.5 h / 20 °C 4: dimethylsulfoxide / 87 h / 80 °C 5: neutral Al2O3 (activity III) View Scheme | |
| Multi-step reaction with 4 steps 1.1: BH3*THF / tetrahydrofuran / -20 °C 1.2: 48 percent / lipase PS; vinyl acetate / various solvent(s) / 72 h / 37 °C 2.1: K2CO3 / methanol / 2.5 h / 20 °C 3.1: dimethylsulfoxide / 87 h / 80 °C 4.1: neutral Al2O3 (activity III) View Scheme |
-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

-
-
408497-91-6
N-(2-Benzyloxy-5-{(R)-1-hydroxy-2-[(R)-2-(4-methoxy-phenyl)-1-methyl-ethylamino]-ethyl}-phenyl)-formamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 87 percent / BH3*THF / tetrahydrofuran / -20 °C 2: lipase PS Celite-immobilized / various solvent(s) / 72 h / 37 °C 3: K2CO3 / methanol / 2.5 h / 20 °C 4: dimethylsulfoxide / 87 h / 80 °C 5: neutral Al2O3 (activity III) 6: 67 percent / Fe turnings; 1M aq. HCl / methanol / 0.75 h / Heating 7: 69 percent / pyridine / 6.5 h / 60 °C View Scheme | |
| Multi-step reaction with 6 steps 1.1: BH3*THF / tetrahydrofuran / -20 °C 1.2: 48 percent / lipase PS; vinyl acetate / various solvent(s) / 72 h / 37 °C 2.1: K2CO3 / methanol / 2.5 h / 20 °C 3.1: dimethylsulfoxide / 87 h / 80 °C 4.1: neutral Al2O3 (activity III) 5.1: 67 percent / Fe turnings; 1M aq. HCl / methanol / 0.75 h / Heating 6.1: 69 percent / pyridine / 6.5 h / 60 °C View Scheme |
-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 87 percent / BH3*THF / tetrahydrofuran / -20 °C 2: lipase PS Celite-immobilized / various solvent(s) / 72 h / 37 °C 3: K2CO3 / methanol / 2.5 h / 20 °C 4: dimethylsulfoxide / 87 h / 80 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: BH3*THF / tetrahydrofuran / -20 °C 1.2: 48 percent / lipase PS; vinyl acetate / various solvent(s) / 72 h / 37 °C 2.1: K2CO3 / methanol / 2.5 h / 20 °C 3.1: dimethylsulfoxide / 87 h / 80 °C View Scheme |
-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

-
-
67346-50-3
(S,R)-Formoterol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 98 percent / oxazaborolidine from cis (1S,2R)-aminoindanol and trimethylboroxine, BH3 / tetrahydrofuran / -15 °C 2: 98 percent / aqueous sodium hydroxide / methanol / 0.5 h 3: 90 °C 4: H2 / PtO2 6: H2 / Pd-C / ethanol View Scheme |
-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

-
-
67346-51-4
N-(2-Hydroxy-5-{(R)-1-hydroxy-2-[(S)-2-(4-methoxy-phenyl)-1-methyl-ethylamino]-ethyl}-phenyl)-formamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 98 percent / oxazaborolidine from cis (1R,2S)-aminoindanol and trimethylboroxine, BH3 / tetrahydrofuran / -15 °C 2: 98 percent / aqueous sodium hydroxide / methanol / 0.5 h 3: 90 °C 4: H2 / PtO2 6: H2 / Pd-C / ethanol View Scheme |
-
-
43229-01-2
4-benzyloxy-3-nitrophenacyl bromide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 98 percent / oxazaborolidine from cis (1S,2R)-aminoindanol and trimethylboroxine, BH3 / tetrahydrofuran / -15 °C 2: 98 percent / aqueous sodium hydroxide / methanol / 0.5 h 3: 90 °C 4: H2 / PtO2 6: H2 / Pd-C / ethanol View Scheme |
2-Bromo-4'-Benzyloxy-3'-nitroacetophenone Chemical Properties
Molecular Structure of 2-Bromo-4'-Benzyloxy-3'-nitroacetophenone (CAS NO.43229-01-2):
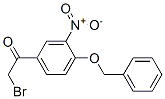
IUPAC: 1-[4-(Benzyloxy)-3-nitrophenyl]-2-bromoethanone
Molecular Formula: C15H12BrNO4
Molecular Weight: 350.16
Density: 1.518 g/cm3
Flash Point: 235.168oC
Boiling Point: 465.239oC at 760 mmHg
Index of Refraction: 1.627
Molar Volume: 230.691 cm3
Surface Tension: 55.231 dyne/cm
Enthalpy of Vaporization: 72.681 kJ/mol
SMILES: BrCC(=O)c2ccc(OCc1ccccc1)c(c2)N(=O)=O
InChI: InChI=1/C15H12BrNO4/c16-9-14(18)12-6-7-15(13(8-12)17(19)20)21-10-11-4-2-1-3-5-11/h1-8H,9-10H2
InChIKey: PBAAKBQGBSUCTG-UHFFFAOYAQ
2-Bromo-4'-Benzyloxy-3'-nitroacetophenone Uses
2-Bromo-4'-Benzyloxy-3'-nitroacetophenone (CAS NO. 43229-01-2) is used as a pharmaceutical intermediate .
2-Bromo-4'-Benzyloxy-3'-nitroacetophenone Specification
2-Bromo-4'-Benzyloxy-3'-nitroacetophenone (CAS NO.43229-01-2) is also known as 1-[4-(Benzyloxy)-3-nitrophenyl]-2-bromoethanone ; 2-Bromo-1-[3-nitro-4-(phenylmethoxy)phenyl]-ethanone ; Ethanone, 2-bromo-1-[3-nitro-4-(phenylmethoxy)phenyl]- ; 2-Bromo-4'-Benzyloxy-3'-nitroacetophenone . This compound should be stored in a cool, dry place when not in use.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View