-
Name
2-Fluoro-4-hydroxybenzoic acid
- EINECS 613-746-2
- CAS No. 65145-13-3
- Article Data10
- CAS DataBase
- Density 1.492 g/cm3
- Solubility
- Melting Point 199-202 °C
- Formula C7H5FO3
- Boiling Point 334.6 °C at 760 mmHg
- Molecular Weight 156.113
- Flash Point 156.2 °C
- Transport Information
- Appearance
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2-Fluoro-4-hydroxybenzoicacid;4-Hydroxy-2-fluorobenzoic acid;
- PSA 57.53000
- LogP 1.22950
Synthetic route
-
-
82380-18-5
2-fluoro-4-hydroxybenzonitrile

-
-
65145-13-3
2-fluoro-4-hydroxybenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2-fluoro-4-hydroxybenzonitrile With sodium hydroxide In water for 4h; Heating / reflux; Stage #2: With hydrogenchloride In water at 20℃; | 100% |
| With sodium hydroxide In water for 60h; Yield given; | |
| With potassium hydroxide In ethanol; water |

-
-
65145-13-3
2-fluoro-4-hydroxybenzoic acid

| Conditions | Yield |
|---|---|
| With boron tribromide In dichloromethane at -78 - 20℃; | 89% |
-
-
860296-15-7
3-chloro-2-fluoro-4-hydroxybenzoic acid

-
-
65145-13-3
2-fluoro-4-hydroxybenzoic acid

| Conditions | Yield |
|---|---|
| With ammonium formate; palladium on activated charcoal In methanol at 25℃; for 20h; | 89% |
-
-
860296-18-0
3-(fluorophenyloxy)triisopropylsilane

-
-
124-38-9
carbon dioxide

-
A
-
65145-13-3
2-fluoro-4-hydroxybenzoic acid

-
B
-
67531-86-6
6-fluorosalicylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 3-(fluorophenyloxy)triisopropylsilane With N,N,N',N'',N'''-pentamethyldiethylenetriamine; sec.-butyllithium In tetrahydrofuran at -75℃; for 2h; Stage #2: carbon dioxide In tetrahydrofuran | A 51% B 5% |

-
-
1583-58-0
2,4-difluoro-benzoic acid

-
A
-
345-29-9
4-fluorosalicylic acid

-
B
-
65145-13-3
2-fluoro-4-hydroxybenzoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide for 7h; Heating; Title compound not separated from byproducts; |

-
-
65145-13-3
2-fluoro-4-hydroxybenzoic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 25℃; for 6h; | 3.24 g |
-
-
124-38-9
carbon dioxide

-
-
146746-69-2
3-(methoxymethoxy)-2-(trimethylsilyl)fluorobenzene

-
-
65145-13-3
2-fluoro-4-hydroxybenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 3-(methoxymethoxy)-2-(trimethylsilyl)fluorobenzene With n-butyllithium; potassium tert-butylate In tetrahydrofuran; hexane at -75℃; for 2h; Stage #2: carbon dioxide In tetrahydrofuran; hexane Stage #3: With hydrogenchloride In water at 25℃; for 6h; |
-
-
860296-13-5
2-chloro-1-fluoro-3-(methoxymethoxy)benzene

-
-
65145-13-3
2-fluoro-4-hydroxybenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: BuLi; 2,2,6,6-tetramethylpiperidine / tetrahydrofuran; hexane / 2 h / -75 °C 1.2: tetrahydrofuran; hexane 1.3: 73 percent / HCl / H2O / pH 1 2.1: 89 percent / ammonium formate / Pd/C / methanol / 20 h / 25 °C View Scheme |
-
-
372-20-3
3-fluorophenol

-
-
65145-13-3
2-fluoro-4-hydroxybenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 83 percent / imidazole / dimethylformamide / 20 h / 25 °C 2.1: sec-BuLi; PMDTA / tetrahydrofuran / 2 h / -75 °C 2.2: 51 percent / tetrahydrofuran View Scheme | |
| Multi-step reaction with 3 steps 1: 61 percent / bromine / CHCl3 / 0.01 h 2: 1-methyl-2-pyrrolidinone / 2 h / 180 °C 3: sodium hydroxide / H2O / 60 h View Scheme |
-
-
126940-10-1
1-fluoro-3-(methoxy-methoxy)benzene

-
-
65145-13-3
2-fluoro-4-hydroxybenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sec-BuLi / tetrahydrofuran; cyclohexane / 2 h / -75 °C 1.2: 86 percent / tetrahydrofuran; cyclohexane / 0.75 h / -75 °C 2.1: BuLi; KOtBu / tetrahydrofuran; hexane / 2 h / -75 °C 2.2: tetrahydrofuran; hexane 3.1: 3.24 g / HCl / H2O / 6 h / 25 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: sec-BuLi / tetrahydrofuran; cyclohexane / 2 h / -75 °C 1.2: 86 percent / tetrahydrofuran; cyclohexane / 0.75 h / -75 °C 2.1: K-OtBu; BuLi / tetrahydrofuran; hexane / 2 h / -75 °C 2.2: tetrahydrofuran; hexane 2.3: HCl / H2O / 6 h / 25 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: sec-BuLi / tetrahydrofuran; cyclohexane / 2 h / -75 °C 1.2: 83 percent / 1,1,2-trichloro-1,2,2-trifluoroethane / tetrahydrofuran; cyclohexane / 0.75 h / -75 °C 2.1: BuLi; 2,2,6,6-tetramethylpiperidine / tetrahydrofuran; hexane / 2 h / -75 °C 2.2: tetrahydrofuran; hexane 2.3: 73 percent / HCl / H2O / pH 1 3.1: 89 percent / ammonium formate / Pd/C / methanol / 20 h / 25 °C View Scheme |
-
-
146746-69-2
3-(methoxymethoxy)-2-(trimethylsilyl)fluorobenzene

-
-
65145-13-3
2-fluoro-4-hydroxybenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: BuLi; KOtBu / tetrahydrofuran; hexane / 2 h / -75 °C 1.2: tetrahydrofuran; hexane 2.1: 3.24 g / HCl / H2O / 6 h / 25 °C View Scheme |
-
-
121219-03-2
4-bromo-3-fluorophenol

-
-
65145-13-3
2-fluoro-4-hydroxybenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1-methyl-2-pyrrolidinone / 2 h / 180 °C 2: sodium hydroxide / H2O / 60 h View Scheme |

-
-
65145-13-3
2-fluoro-4-hydroxybenzoic acid
2-Fluoro-4-hydroxybenzoic acid Specification
The Benzoicacid, 2-fluoro-4-hydroxy-, with the CAS registry number 65145-13-3, is also known as 2-Fluoro-4-hydroxybenzoicacid. It belongs to the product categories of Fine Chemical & Intermediates; Aromatic Carboxylic Acids, Amides, Anilides, Anhydrides & Salts; Benzoic acid; Acids & Esters; Fluorine Compounds; Phenols. This chemical's molecular formula is C7H5FO3 and molecular weight is 156.11. Its systematic name is called 2-fluoro-4-hydroxybenzoic acid. The product should be sealed and stored in cool and dry place. What's more, it should be protected from strong oxides.
Physical properties of Benzoicacid, 2-fluoro-4-hydroxy-: (1)ACD/LogP: 1.78; (2)ACD/LogD (pH 5.5): -0.19; (3)ACD/LogD (pH 7.4): -1.37; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 2.4; (7)ACD/KOC (pH 7.4): 1; (8)#H bond acceptors: 3; (9)#H bond donors: 2; (10)#Freely Rotating Bonds: 2; (11)Index of Refraction: 1.585; (12)Molar Refractivity: 35.05 cm3; (13)Molar Volume: 104.6 cm3; (14)Surface Tension: 60.3 dyne/cm; (15)Density: 1.492 g/cm3; (16)Flash Point: 156.2 °C; (17)Enthalpy of Vaporization: 60.96 kJ/mol; (18)Boiling Point: 334.6 °C at 760 mmHg; (19)Vapour Pressure: 4.99E-05 mmHg at 25°C.
Preparation of Benzoicacid, 2-fluoro-4-hydroxy-: this chemical can be prepared by 2-fluoro-4-propoxybenzoic acid. This reaction will need reagent BBr3 and solvent CH2Cl2. The reaction temperature is -78 - 20 °C. The yield is about 89%.
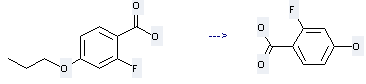
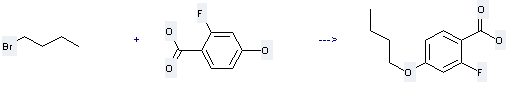
Uses of Benzoicacid, 2-fluoro-4-hydroxy-: it can be used to produce 4-butoxy-2-fluorobenzoic acid with 1-bromo-butane by heating. This reaction will need reagents ethanol, potassium hidroxide and solvent H2O with reaction time of 25 hours. The yield is about 64%.
When you are using this chemical, please be cautious about it as the following:
This chemical may cause inflammation to the skin or other mucous membranes. It is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. Whenever you will contact it, please wear suitable protective clothing.
You can still convert the following datas into molecular structure:
(1)SMILES: Fc1c(C(=O)O)ccc(O)c1
(2)InChI: InChI=1/C7H5FO3/c8-6-3-4(9)1-2-5(6)7(10)11/h1-3,9H,(H,10,11)
(3)InChIKey: NXWTWYULZRDBSA-UHFFFAOYAX
Related Products
- 2-Fluoro-4-hydroxybenzoic acid
- 65146-94-3
- 65147-06-0
- 65147-22-0
- 65148-10-9
- 6515-09-9
- 65151-34-0
- 65151-76-0
- 65151-85-1
- 6515-38-4
- 651558-58-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View