-
Name
2-Fluoroethanol
- EINECS 206-740-2
- CAS No. 371-62-0
- Article Data32
- CAS DataBase
- Density 0.993 g/cm3
- Solubility
- Melting Point -26.5 °C(lit.)
- Formula C2H5FO
- Boiling Point 103.5 °C at 760 mmHg
- Molecular Weight 64.0595
- Flash Point 31.1 °C
- Transport Information UN 3383 6.1/PG 1
- Appearance Colorless Liquid
- Safety 36/37/39-45
- Risk Codes 10-26/27/28
-
Molecular Structure
-
Hazard Symbols
 F;
F;  T+
T+
- Synonyms 1-Fluoroethan-2-ol;Ethylene fluorohydrin;Flutritex 2;NSC 158283;b-Fluoroethanol;
- PSA 20.23000
- LogP -0.05180
2-Fluoroethanol Consensus Reports
2-Fluoroethanol Specification
The IUPAC name of this chemical is 2-Fluoroethanol. With the CAS registry number 371-62-0 and EINECS registry number 206-740-2, it is also named as ethanol,2-fluoro-. In addition, the molecular formula is C2H5FO and the molecular weight is 64.06. It belongs to the classes of Pharmaceutical Intermediates; Alcohols; C2 to C6; Oxygen Compounds. And it should be stored in brown glass bottles, then put in a cool and dry place.
Physical properties about this chemical are: (1)ACD/LogP: -0.47; (2)ACD/LogD (pH 5.5): -0.47; (3)ACD/LogD (pH 7.4): -0.47; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 13.22; (7)ACD/KOC (pH 7.4): 13.22; (8)#H bond acceptors: 1; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 2; (11)Polar Surface Area: 9.23 Å2; (12)Index of Refraction: 1.325; (13)Molar Refractivity: 13.01 cm3; (14)Molar Volume: 64.5 cm3; (15)Polarizability: 5.15 ×10-24cm3; (16)Surface Tension: 20.9 dyne/cm; (17)Density: 0.993 g/cm3; (18)Flash Point: 31.1 °C; (19)Enthalpy of Vaporization: 39.94 kJ/mol; (20)Boiling Point: 103.5 °C at 760 mmHg; (21)Vapour Pressure: 17.8 mmHg at 25°C.
Preparation of 2-Fluoroethanol: it can be prepared by 2-fluoroethyl fluorosulfite. This reaction will need reagent 15percent KOH and solvent H2O. The reaction time is 2 hours. The yield is about 90%.
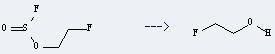
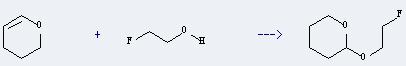
Uses of 2-Fluoroethanol: it is used as pesticides and medicine intermediates. And it can react with 3,4-dihydro-2H-pyran to get 2-(2'-fluoroethoxy)tetrahydropyran. This reaction will need reagent conc.HCl. The reaction time is 3 hours. The yield is about 52%.
When you are using this chemical, please be cautious about it as the following:
This chemical is flammable. And it is very toxic by inhalation, in contact with skin and if swallowed. During using it, wear suitable protective clothing, gloves and eye/face protection. In case of accident or if you feel unwell, seek medical advice immediately (show label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: FCCO
(2)InChI: InChI=1/C2H5FO/c3-1-2-4/h4H,1-2H2
(3)InChIKey: GGDYAKVUZMZKRV-UHFFFAOYAT
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| cat | LC50 | inhalation | 35mg/m3/10M (35mg/m3) | BEHAVIORAL: COMA LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS | National Technical Information Service. Vol. PB158-508, |
| dog | LC50 | inhalation | 7mg/m3/10M (7mg/m3) | BEHAVIORAL: COMA LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS | National Technical Information Service. Vol. PB158-508, |
| guinea pig | LC50 | inhalation | 150mg/m3/10M (150mg/m3) | National Technical Information Service. Vol. PB158-508, | |
| monkey | LC50 | inhalation | 1500mg/m3/10M (1500mg/m3) | BEHAVIORAL: COMA LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS | National Technical Information Service. Vol. PB158-508, |
| mouse | LC50 | inhalation | 1100mg/m3/10M (1100mg/m3) | Journal of the Chemical Society. Vol. -, Pg. 773, 1949. | |
| mouse | LD50 | intraperitoneal | 10mg/kg (10mg/kg) | Journal of Organic Chemistry. Vol. 21, Pg. 739, 1956. | |
| mouse | LD50 | subcutaneous | 15mg/kg (15mg/kg) | Nature. Vol. 172, Pg. 1139, 1953. | |
| rabbit | LC50 | inhalation | 25mg/m3/10M (25mg/m3) | BEHAVIORAL: COMA GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION | National Technical Information Service. Vol. PB158-508, |
| rabbit | LDLo | intravenous | 250ug/kg (0.25mg/kg) | Journal of the Chemical Society. Vol. -, Pg. 1279, 1949. | |
| rat | LC50 | inhalation | 200mg/m3/10M (200mg/m3) | National Technical Information Service. Vol. PB158-508, | |
| rat | LD50 | intraperitoneal | 1750ug/kg (1.75mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Journal of Pharmacy and Pharmacology. Vol. 20, Pg. 465, 1968. |
| rat | LD50 | oral | 5mg/kg (5mg/kg) | "Prehled Prumyslove Toxikologie; Organicke Latky," Marhold, J., Prague, Czechoslovakia, Avicenum, 1986Vol. -, Pg. 515, 1986. |
Related Products
- 2-Fluoroethanol
- 37167-59-2
- 37167-62-7
- 37169-66-7
- 37172-53-5
- 3717-28-0
- 3717-38-2
- 3717-44-0
- 37174-65-5
- 3717-48-4
- 371758-72-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View