-
Name
ALPHA-PYRONE
- EINECS 207-990-5
- CAS No. 504-31-4
- Article Data30
- CAS DataBase
- Density 1.192 g/cm3
- Solubility
- Melting Point 8-9 °C
- Formula C5H4O2
- Boiling Point 207.5 °C at 760 mmHg
- Molecular Weight 96.0856
- Flash Point 89.2 °C
- Transport Information
- Appearance Clear yellow to brown liquid
- Safety 23-24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 1,2-Pyrone(4CI);2,4-Pentadienoic acid, 5-hydroxy-, d-lactone;2-Pyranone;2-Pyrone;2H-Pyran, 2-oxo-;Coumalin;
- PSA 30.21000
- LogP 0.63980
Synthetic route
-
-
53646-72-3
5-bromo-5,6-dihydro-2H-pyran-2-one

-
-
504-31-4
pyran-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 5 - 40℃; | 89% |
| With triethylamine for 0.5h; Dehydrobromination; Heating; | 70% |
| With triethylamine Yield given; |
-
-
25566-16-9
hexane-3,4-cis-diol-2,5-dione

-
-
504-31-4
pyran-2-one

| Conditions | Yield |
|---|---|
| With sodium dihydrogenphosphate; sodium hydroxide In acetic acid butyl ester; water at 80℃; for 12h; | 79.7% |
-
-
19978-32-6
3-bromo-2H-pyran-2-one

-
-
15681-48-8
lithium dimethylcuprate

-
A
-
31678-73-6
3-methyl-2H-pyran-2-one

-
B
-
504-31-4
pyran-2-one

| Conditions | Yield |
|---|---|
| In diethyl ether at -78℃; for 2h; Yields of byproduct given; | A 5% B n/a |


| Conditions | Yield |
|---|---|
| With copper |

| Conditions | Yield |
|---|---|
| With copper at 650℃; under 5 Torr; |
-
-
1192-27-4
5-diazo-1,3-cyclopentadiene

-
A
-
504-31-4
pyran-2-one

-
B
-
13177-38-3
cyclopentadienone

-
C
-
4729-01-5
cyclopentadienylidene

-
D
-
39763-18-3
(Z)-5-Oxo-penta-2,4-dienal

| Conditions | Yield |
|---|---|
| With oxygen at -253.2℃; Mechanism; Irradiation; other temperatures, different λ, reaction with (18)O(16)O and (18)O2; |

-
-
1192-27-4
5-diazo-1,3-cyclopentadiene

-
A
-
504-31-4
pyran-2-one

-
B
-
13177-38-3
cyclopentadienone

-
C
-
39763-18-3
(Z)-5-Oxo-penta-2,4-dienal

| Conditions | Yield |
|---|---|
| With oxygen In solid matrix at -253.2℃; Product distribution; Mechanism; Irradiation; further with <18O>O2; |


| Conditions | Yield |
|---|---|
| at 620℃; |
-
-
69528-36-5, 127642-28-8
exo-4,5-epoxy-exo-10-oxatricyclo<5.2.1.02,6>deca-8-en-3-one

-
A
-
504-31-4
pyran-2-one

-
-
68781-88-4, 129076-58-0
4,5-epoxy-2-cyclopentenone

| Conditions | Yield |
|---|---|
| under 0.04 Torr; for 1.5h; flash vacuum thermolysis; T1 75 deg C / T2 375 deg C; Yield given; |
-
-
88766-67-0
cyclopentadienone O-oxide

-
A
-
504-31-4
pyran-2-one

-
B
-
13177-38-3
cyclopentadienone

-
C
-
39763-18-3
(Z)-5-Oxo-penta-2,4-dienal

-
D
-
68781-88-4
cyclopentadienone oxide

| Conditions | Yield |
|---|---|
| Mechanism; Irradiation; |


-
-
504-31-4
pyran-2-one

| Conditions | Yield |
|---|---|
| Beim Destillieren im Wasserstoffstrom; |
-
-
10374-07-9
3-oxabicyclo<3.2.0>hept-6-ene-2,4-dione

-
A
-
22980-23-0
2-oxa-3-oxobicyclo[2.2.0]hex-5-ene

-
B
-
504-31-4
pyran-2-one

-
C
-
1120-53-2
1,3-cyclobutadiene

-
D
-
39763-18-3
(Z)-5-Oxo-penta-2,4-dienal

| Conditions | Yield |
|---|---|
| In solid matrix at -269.2 - -248.2℃; Product distribution; Mechanism; Irradiation; irradiation with 248 nm, subsequent bleaching with 254- and 313 nm light destroys part of products; also with isotopomers; |

-
-
10374-07-9
3-oxabicyclo<3.2.0>hept-6-ene-2,4-dione

-
A
-
689-97-4
3-buten-1-yne

-
B
-
504-31-4
pyran-2-one

-
C
-
13177-38-3
cyclopentadienone

| Conditions | Yield |
|---|---|
| at 850℃; Product distribution; detection on argon matrix, 10 K; |

-
-
60249-17-4
6-methoxy-2H-pyran-3(6H)-one

-
-
504-31-4
pyran-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 90 percent / LiAlH4 / diethyl ether / 0.5 h / -60 °C 2: 72 percent / NBS; Me2S / CH2Cl2 / 1 h / 0 °C 3: 90 percent / Jones reagent / acetone / 0.5 h 4: 70 percent / Et3N / 0.5 h / Heating View Scheme |
-
-
35436-57-8
6-hydroxy-3,6-dihydro-2H-pyran-3-one

-
-
504-31-4
pyran-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 75 percent / Ag2O / acetone / 24 h / 20 °C 2: 90 percent / LiAlH4 / diethyl ether / 0.5 h / -60 °C 3: 72 percent / NBS; Me2S / CH2Cl2 / 1 h / 0 °C 4: 90 percent / Jones reagent / acetone / 0.5 h 5: 70 percent / Et3N / 0.5 h / Heating View Scheme |
-
-
283156-05-8
methyl 2,3-dideoxy-α,β-DL-glycero-pent-2-enopyranoside

-
-
504-31-4
pyran-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 72 percent / NBS; Me2S / CH2Cl2 / 1 h / 0 °C 2: 90 percent / Jones reagent / acetone / 0.5 h 3: 70 percent / Et3N / 0.5 h / Heating View Scheme |
-
-
283156-10-5
3-bromo-6-methoxy-3,6-dihydro-2H-pyran

-
-
504-31-4
pyran-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 90 percent / Jones reagent / acetone / 0.5 h 2: 70 percent / Et3N / 0.5 h / Heating View Scheme |
-
-
64919-86-4, 69609-00-3
exo-10-oxatricyclo<5.2.1.02,6>deca-4,8-dien-3-one

-
-
504-31-4
pyran-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 94 percent / 33percent hydrogen peroxide, 0.2 N sodium hydroxide / methanol; CH2Cl2; H2O / 3 h 2: 1.5 h / 0.04 Torr / flash vacuum thermolysis; T1 75 deg C / T2 375 deg C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 6 percent / piridinium dichromate / CH2Cl2 / 2 h / 0 °C 2: 0.025 g / triethylamine / toluene / 0.5 h / 80 °C 3: NBS 4: triethylamine View Scheme | |
| Multi-step reaction with 3 steps 1: 50 percent / piridinium dichromate / CH2Cl2 / 2 h / 0 °C 2: NBS 3: triethylamine View Scheme | |
| Multi-step reaction with 4 steps 1: 6 percent / piridinium dichromate, t-butyl hydroperoxide / CH2Cl2 / 2 h / 0 °C 2: 0.025 g / triethylamine / toluene / 0.5 h / 80 °C 3: NBS 4: triethylamine View Scheme | |
| Multi-step reaction with 3 steps 1: 50 percent / piridinium dichromate, t-butyl hydroperoxide / CH2Cl2 / 2 h / 0 °C 2: NBS 3: triethylamine View Scheme |
-
-
70411-95-9
2-(tert.butylperoxy)-3,4-dihydropyran

-
-
504-31-4
pyran-2-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 0.025 g / triethylamine / toluene / 0.5 h / 80 °C 2: NBS 3: triethylamine View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NBS 2: triethylamine View Scheme |
-
-
504-31-4
pyran-2-one

-
-
22980-23-0
5-oxabicyclo<2.2.0>hex-2-en-6-one

| Conditions | Yield |
|---|---|
| In diethyl ether at -15℃; Inert atmosphere; Irradiation; | 100% |
| In diethyl ether at -10℃; for 24h; Inert atmosphere; Irradiation; | 100 %Spectr. |
| In diethyl ether at -10℃; UV-irradiation; Inert atmosphere; | |
| In diethyl ether at -10℃; Inert atmosphere; Microwave irradiation; | |
| In diethyl ether at -15℃; Inert atmosphere; Irradiation; |
-
-
504-31-4
pyran-2-one

| Conditions | Yield |
|---|---|
| In hexane ligand was added to hexane soln. of Os-cluster in Schlenk tube, heated at 40°C for 2 h under N2; solvent was removed under reduced pressure, recrystd. from CH2Cl2/hexaneat -5°C; elem. anal.; | 99% |
-
-
941-69-5
N-phenyl-maleimide

-
-
504-31-4
pyran-2-one

-
-
64856-70-8, 97643-47-5
N,N'-diphenylbicyclo<2.2.2>oct-7-ene-2,3,5,6-tetracarboxydiimide

| Conditions | Yield |
|---|---|
| In decalin for 1.5h; Heating; | 98% |
-
-
504-31-4
pyran-2-one

-
-
22682-17-3
5,6-dibromo-5,6-dihydro-2H-pyran-2-one

| Conditions | Yield |
|---|---|
| Stage #1: pyran-2-one With bromine In dichloromethane at -78℃; for 5h; Inert atmosphere; Irradiation; Stage #2: In dichloromethane at -78 - 20℃; Inert atmosphere; | 97% |
| With bromine In dichloromethane at -78℃; for 6h; Cooling with acetone-dry ice; Irradiation; | 97% |
| With bromine Irradiation; |

| Conditions | Yield |
|---|---|
| In 1,2-dimethoxyethane at 60℃; for 4h; Inert atmosphere; | 97% |
-
-
504-31-4
pyran-2-one

-
-
999-55-3
allyl acrylate

-
-
134870-02-3, 134932-34-6, 144489-99-6, 144490-01-7
3-Oxo-2-oxa-bicyclo[2.2.2]oct-7-ene-6-carboxylic acid allyl ester

| Conditions | Yield |
|---|---|
| under 14251100 Torr; for 24h; | 95% |
-
-
504-31-4
pyran-2-one

-
-
3085-68-5
N,N-diallylacrylamide

-
-
134870-04-5, 134932-36-8
3-Oxo-2-oxa-bicyclo[2.2.2]oct-7-ene-6-carboxylic acid diallylamide

| Conditions | Yield |
|---|---|
| under 14251100 Torr; for 24h; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: pyran-2-one With 2,2,6,6-tetramethylpiperidinylmagnesium chloride lithium chloride complex In tetrahydrofuran at -40℃; for 0.25h; Schlenk technique; Inert atmosphere; Stage #2: methanethiosulfonic acid S-methyl ester In tetrahydrofuran at -40 - 25℃; Schlenk technique; Inert atmosphere; regioselective reaction; | 95% |
-
-
504-31-4
pyran-2-one

-
-
22980-23-0
2-oxa-3-oxobicyclo[2.2.0]hex-5-ene

| Conditions | Yield |
|---|---|
| In diethyl ether at -15℃; UV-irradiation; | 95% |
-
-
504-31-4
pyran-2-one

-
-
85263-68-9
3,3,4,4,7,7,8,8-octamethyl-3,4,7,8-tetrasilylcycloocta-1,5-diyne

-
-
85263-70-3
1,1,2,2,9,9,10,10-octamethyl-1,2,9,10-tetrasila<2.2>ortocyclophane

| Conditions | Yield |
|---|---|
| With triethylamine In toluene | 93% |
-
-
108-31-6
maleic anhydride

-
-
504-31-4
pyran-2-one

-
-
1515-21-5, 26290-47-1
3-Oxo-2-oxabicylo<2.2.2>oct-7-en-exo-5,exo-6-dicarbonsaeureanhydrid

| Conditions | Yield |
|---|---|
| In toluene at 55℃; under 3375270 Torr; for 12h; | 92% |
| In toluene at 20 - 110℃; Diels-Alder reaction; | 79% |
| In xylene at 90.5℃; Rate constant; Thermodynamic data; ΔH(excit.), ΔS(excit.); further temp.; | |
| With toluene |

| Conditions | Yield |
|---|---|
| With (+)-Yb(tfc)3 In neat (no solvent) for 72h; Product distribution; Ambient temperature; further Lewis acids, also in solvent; other benzylic vinyl ether; variation of excess of benzyl vinyl ether; | 91% |
| With (+)-Yb(tfc)3 In neat (no solvent) for 72h; Ambient temperature; | 91% |
-
-
504-31-4
pyran-2-one

-
-
78129-68-7
2,2-dimethylpropylidynephosphine

-
-
109827-19-2
2-tert-Butyl-λ3-phosphinin

| Conditions | Yield |
|---|---|
| In benzene at 120℃; under 1875.1 - 2250.2 Torr; for 48h; | 91% |

| Conditions | Yield |
|---|---|
| for 72h; Ambient temperature; | 88% |

| Conditions | Yield |
|---|---|
| With (+)-Yb(tfc)3 In dichloromethane under 9000720 Torr; for 72h; Ambient temperature; | 88% |
-
-
504-31-4
pyran-2-one

-
-
627-63-4
fumaryl dichloride

-
-
82313-26-6
3-Oxo-2-oxabicyclo<2.2.2>oct-7-en-endo-5,exo-6-bis(carbonylchlorid)

| Conditions | Yield |
|---|---|
| In toluene at 60℃; under 4125330 Torr; for 12h; | 86% |
-
-
504-31-4
pyran-2-one

-
-
51447-09-7
endo-tricyclo<4.2.2.02,5>deca-3,9-diene-7,8-dicarboxylate anhydride

-
-
77884-58-3
C28H24O6

| Conditions | Yield |
|---|---|
| In xylene for 2h; Heating; | 86% |
-
-
504-31-4
pyran-2-one

-
-
159087-46-4
4,4,5,5-tetramethyl-2-trimethylsilanylethynyl-[1,3,2]dioxaborolane

-
-
910875-07-9
trimethyl(2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)silane

| Conditions | Yield |
|---|---|
| at 170℃; for 15h; | 86% |
| In neat (no solvent) 1.5 equiv. of B compd., sealed, heated at 170°C for 17 h; | 86% |
| In 1,3,5-trimethyl-benzene 1.5 equiv. of B compd., sealed, heated at 170°C for 17 h; | 64% |
| In 1,3,5-trimethyl-benzene at 140°C for 48 h; | 56% |
| In diphenylether 1.5 equiv. of B compd., sealed, heated at 170°C for 17 h; | 56% |
-
-
67969-82-8
tetrafluoroboric acid diethyl ether

-
-
504-31-4
pyran-2-one

| Conditions | Yield |
|---|---|
| In dichloromethane soln. of complex cooled to -80°C, addn. of B-compd. with stirring, addn. of ligand after 5 min, warmed to 0°C over the course of 2h, kept at 0°C for 1h; concd. (vac.), dropwise addn. of ether, filtered, washed (ether), dried (vac.); elem. anal.; | 86% |


| Conditions | Yield |
|---|---|
| With iron(III)-acetylacetonate In diethyl ether at -30℃; Inert atmosphere; | 86% |
-
-
941-69-5
N-phenyl-maleimide

-
-
504-31-4
pyran-2-one

-
-
64856-70-8, 97643-47-5
endo,endo-bis-N-phenylimide of bicyclo<2.2.2>oct-7-ene-2,3,5,6-tetracarboxylic acid

| Conditions | Yield |
|---|---|
| In xylene at 140℃; | 85% |
-
-
504-31-4
pyran-2-one

-
-
1121707-11-6
1-methyl-4-(trimethylsilyl)-1H-indol-5-yl trifluoromethanesulfonate

-
-
23840-48-4
3-methyl-3H-benzo[e]indole

| Conditions | Yield |
|---|---|
| With cesium fluoride In acetonitrile at 100℃; for 5h; Inert atmosphere; | 85% |
| With cesium fluoride In acetonitrile at 100℃; | 85% |
-
-
504-31-4
pyran-2-one

-
-
154920-12-4
hept-6-en-1-ylmagnesium bromide

-
-
1417889-31-6
(-)-(R)-4-(hept-6-en-1-yl)-3,4-dihydro-2H-pyran-2-one

| Conditions | Yield |
|---|---|
| Stage #1: hept-6-en-1-ylmagnesium bromide With copper(I) bromide dimethylsulfide complex; (R,S)-reverse-Josiphos In diethyl ether; tert-butyl methyl ether at -72℃; for 0.25h; Inert atmosphere; Schlenk technique; Stage #2: pyran-2-one In diethyl ether; tert-butyl methyl ether at -72℃; Inert atmosphere; Schlenk technique; enantioselective reaction; | 85% |

| Conditions | Yield |
|---|---|
| at 200℃; for 2h; | 84% |
-
-
504-31-4
pyran-2-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 20℃; for 12h; Inert atmosphere; | 82% |
-
-
504-31-4
pyran-2-one

-
-
74844-06-7
(3-methylphenyl)(6-methyl-2-pyridyl)acetylene

-
-
74844-07-8
1-(3-methylphenyl)-2-(6-methyl-2-pyridyl)benzene

| Conditions | Yield |
|---|---|
| With hydroquinone In 1,2-dichloro-benzene for 72h; Heating; | 80% |
2H-Pyran-2-one Specification
The CAS register number of 2H-Pyran-2-one is 504-31-4. It also can be called as 2-Pyranone and the IUPAC name about this chemical is pyran-2-one. The molecular formula about this chemical is C5H4O2 and the molecular weight is 96.08.
Physical properties about 2H-Pyran-2-one are: (1)ACD/LogP: -0.21; (2)ACD/LogD (pH 5.5): -0.2; (3)ACD/LogD (pH 7.4): -0.2; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 18.44; (7)ACD/KOC (pH 7.4): 18.44; (8)#H bond acceptors: 2; (9)Polar Surface Area: 26.3 Å2; (10)Index of Refraction: 1.507; (11)Molar Refractivity: 24 cm3; (12)Molar Volume: 80.5 cm3; (13)Polarizability: 9.51x10-24cm3; (14)Surface Tension: 38.9 dyne/cm; (15)Density: 1.192 g/cm3; (16)Flash Point: 89.2 °C; (17)Enthalpy of Vaporization: 44.38 kJ/mol; (18)Boiling Point: 207.5 °C at 760 mmHg; (19)Vapour Pressure: 0.225 mmHg at 25 °C.
Preparation: this chemical can be prepared by 5-bromo-5,6-dihydro-pyran-2-one. This reaction will need reagent of triethylamine.

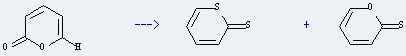
Uses of 2H-Pyran-2-one: it can be used to produce thiopyran-2-thione and pyran-2-one. This reaction will need reagent of Lawesson's reagent and solvent of benzene. The reaction time is 40 hours. The yield is about 11%.
When you are using this chemical, please be cautious about it as the following:
This chemical is harmful to the environment and it can pollute the water, do not put materials into the water. If you want to store it, you should keep the container tightly sealed in dry, cool places and avoid contact with oxide, source of ignition. It is stable under normal temperature and pressure. If you want to use this chemical, do not breathe vapour and avoid contact with skin and eyes.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C\1O\C=C/C=C/1
(2)InChI: InChI=1/C5H4O2/c6-5-3-1-2-4-7-5/h1-4H
(3)InChIKey: ZPSJGADGUYYRKE-UHFFFAOYAI
(4)Std. InChI: InChI=1S/C5H4O2/c6-5-3-1-2-4-7-5/h1-4H
(5)Std. InChIKey: ZPSJGADGUYYRKE-UHFFFAOYSA-N
Related Products
- 2H-Pyran-2-one
- 2H-Pyran-2-one, 4,6-diphenyl-
- 2H-Pyran-2-one, 6-chloro-
- 2H-Pyran-2-one,3,3'-methylenebis[6-ethyl-4-hydroxy-5-methyl-
- 2H-Pyran-2-one,3,4-dihydro-6-methyl-
- 2H-Pyran-2-one,3,6-dihydro-4,6,6-trimethyl-
- 2H-Pyran-2-one,3-ethyltetrahydro-
- 2H-Pyran-2-one,4-hydroxy-3,6-dimethyl-
- 2H-Pyran-2-one,5,6-dihydro-4-hydroxy-6-methyl-
- 2H-Pyran-2-one,5,6-dihydro-6-pentyl-
- 50432-68-3
- 50432-79-6
- 50433-06-2
- 504-33-6
- 50434-36-1
- 50436-33-4
- 5043-81-2
- 50439-35-5
- 50439-37-7
- 50439-45-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View