-
Name
3,5-Dimethoxybenzyl alcohol
- EINECS 211-888-6
- CAS No. 705-76-0
- Article Data127
- CAS DataBase
- Density 1.111 g/cm3
- Solubility Insoluble in water
- Melting Point 46-50 °C
- Formula C9H12O3
- Boiling Point 299.1 °C at 760 mmHg
- Molecular Weight 168.192
- Flash Point 134.7 °C
- Transport Information
- Appearance White to yellow-beige crystalline solid
- Safety 22-24/25-36/37-26
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Benzylalcohol, 3,5-dimethoxy- (7CI,8CI);(3,5-Dimethoxyphenyl)methan-1-ol;(3,5-Dimethoxyphenyl)methanol;AC1L2CI8;AC1Q56CZ;
- PSA 38.69000
- LogP 1.19610
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol at 20℃; for 0.166667h; | 100% |
| With sodium tetrahydroborate In methanol at 20℃; for 1h; | 100% |
| With sodium tetrahydroborate In ethanol at 20℃; for 0.5h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride; 4-methyltetrahydropyran at 0 - 20℃; for 1h; Inert atmosphere; Green chemistry; | 100% |
| With lithium aluminium tetrahydride In tetrahydrofuran | 99% |
| With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 20℃; for 1.33333h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol; 1,2-dimethoxyethane for 3h; Heating; | 99% |
| With lithium aluminium tetrahydride In diethyl ether at 0℃; for 1h; | 99% |
| With lithium aluminium tetrahydride In diethyl ether Heating; | 98% |

-
-
705-76-0
3,5-dimethoxybenzyl alcohol

| Conditions | Yield |
|---|---|
| With zinc trifluoromethanesulfonate In methanol at 0 - 20℃; for 0.666667h; chemoselective reaction; | 98% |
-
-
1523213-10-6
3,5-dimethoxy((triethylsilyloxy)methyl)benzene

-
-
705-76-0
3,5-dimethoxybenzyl alcohol

| Conditions | Yield |
|---|---|
| With zinc trifluoromethanesulfonate In methanol at 0 - 20℃; for 0.25h; chemoselective reaction; | 91% |

-
-
705-76-0
3,5-dimethoxybenzyl alcohol

| Conditions | Yield |
|---|---|
| With zinc trifluoromethanesulfonate In methanol at 0 - 20℃; for 1h; chemoselective reaction; | 89% |
-
-
50-00-0
formaldehyd

-
-
192182-54-0
3,5-dimethoxyphenylboronic acid

-
-
705-76-0
3,5-dimethoxybenzyl alcohol

| Conditions | Yield |
|---|---|
| With bis(η3-allyl-μ-chloropalladium(II)); 1-(2-bromophenyl)-3-(2,6-diisopropylphenyl)-4,5-dihydroimidazolinium chloride In tetrahydrofuran; water at 100℃; for 2h; Inert atmosphere; Sealed tube; | 86% |
-
-
17275-82-0
ethyl 3,5-dimethoxybenzoate

-
-
705-76-0
3,5-dimethoxybenzyl alcohol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In diethyl ether for 3h; Reduction; Heating; | 84% |
| With lithium aluminium tetrahydride In diethyl ether for 1h; Heating; | |
| With lithium aluminium tetrahydride In tetrahydrofuran at 20℃; for 3h; Inert atmosphere; |
-
-
115130-81-9
(1,1-dimethylethyl)diphenylsilyl 3,5-dimethoxybenzyl ether

-
-
705-76-0
3,5-dimethoxybenzyl alcohol

| Conditions | Yield |
|---|---|
| With zinc trifluoromethanesulfonate In methanol at 0 - 20℃; for 3h; chemoselective reaction; | 84% |

-
-
60319-09-7
3,5-dimethoxyphenyl trifluoromethanesulfonate

-
-
705-76-0
3,5-dimethoxybenzyl alcohol

| Conditions | Yield |
|---|---|
| With sodium carbonate; bis(dibenzylideneacetone)-palladium(0); ruphos In 1,4-dioxane; water for 10h; Suzuki-Miyaura cross-coupling; Inert atmosphere; Reflux; | 80.9% |

| Conditions | Yield |
|---|---|
| With sodium carbonate; bis(dibenzylideneacetone)-palladium(0); ruphos In 1,4-dioxane; water for 48h; Suzuki-Miyaura cross-coupling; Inert atmosphere; Reflux; | 80.9% |
-
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
A
-
705-76-0
3,5-dimethoxybenzyl alcohol

-
B
-
1132-21-4
3,5-dimethoxybenzoic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide at 100℃; for 0.0833333h; solvent-free Cannizzaro reaction; | A 41% B 49% |
| With potassium hydroxide auf dem Dampfbad; |
-
-
17213-58-0
3,5-dimethoxybenzamide

-
-
705-76-0
3,5-dimethoxybenzyl alcohol

| Conditions | Yield |
|---|---|
| With sodium amalgam; ethanol |
-
-
67-56-1
methanol

-
-
53759-16-3
3,5-dimethoxybenzyltrimethylammonium iodide

-
A
-
705-76-0
3,5-dimethoxybenzyl alcohol

-
B
-
4179-19-5
1,3-dimethoxy-5-methylbenzene

-
C
-
20230-89-1
trimethylammonium iodide

-
D
-
51066-74-1
dimethylamine hydriodide

| Conditions | Yield |
|---|---|
| With water at 30℃; for 2h; Irradiation; | A 42 % Chromat. B 31 % Chromat. C 70 % Chromat. D 30 % Chromat. |


| Conditions | Yield |
|---|---|
| With water at 30℃; for 2h; Irradiation; |


| Conditions | Yield |
|---|---|
| With water at 30℃; for 2h; Irradiation; |

-
-
124-38-9
carbon dioxide

-
-
74726-76-4
2-bromo-3,5-dimethoxybenzyl alcohol

-
A
-
3465-69-8
5,7-dimethoxyphthalide

-
B
-
705-76-0
3,5-dimethoxybenzyl alcohol

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction. Yields of byproduct given; |

-
-
132212-93-2
3-(3,5-Dimethoxy-benzyl)-3H-purin-6-ylamine

-
A
-
705-76-0
3,5-dimethoxybenzyl alcohol

| Conditions | Yield |
|---|---|
| In water Mechanism; Product distribution; Quantum yield; Irradiation; other meta-substituted N-arylmethyladenines and also other solvents investigated; |

| Conditions | Yield |
|---|---|
| In water at 30℃; for 2h; Mechanism; Irradiation; >/= 260 nm; |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride; potassium carbonate 1.) acetone; 2.) THF; Multistep reaction; |
-
-
38513-65-4
3,5-dimethoxybenzyl acetate

-
-
705-76-0
3,5-dimethoxybenzyl alcohol

| Conditions | Yield |
|---|---|
| With potassium hydroxide |
-
-
38513-65-4
3,5-dimethoxybenzyl acetate

-
A
-
705-76-0
3,5-dimethoxybenzyl alcohol

-
B
-
51768-56-0
1,3-dimethoxy-5-ethylbenzene

| Conditions | Yield |
|---|---|
| In water Irradiation; Title compound not separated from byproducts; | A 92 % Spectr. B 0.2 % Spectr. |
-
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
-
191607-97-3
O-(4-methoxyphenyl)-N,N-diethylthiocarbamate

-
A
-
705-76-0
3,5-dimethoxybenzyl alcohol

| Conditions | Yield |
|---|---|
| With N,N,N,N,-tetramethylethylenediamine; sec.-butyllithium 1) cyclohexane, THF, -78 deg C, 2 h; 2) cyclohexane, THF, -78 deg C, 1 h; Yield given. Multistep reaction. Yields of byproduct given; |



| Conditions | Yield |
|---|---|
| In acetonitrile at 30℃; Kinetics; Further Variations:; wavelength of UV-light; photolysis; UV-irradiation; |

| Conditions | Yield |
|---|---|
| In acetonitrile at 30℃; Kinetics; photolysis; UV-irradiation; |

-
-
540-63-6
ethane-1,2-dithiol

-
-
388570-72-7
2-(tert-butyl-dimethyl-silanyloxymethyl)-4-hydroxy-6-methoxy-benzaldehyde

-
A
-
705-76-0
3,5-dimethoxybenzyl alcohol

| Conditions | Yield |
|---|---|
| With zinc(II) chloride In dichloromethane |


-
A
-
55-18-5
N-Nitrosodiethylamine

-
B
-
705-76-0
3,5-dimethoxybenzyl alcohol

-
C
-
7311-34-4
3,5-dimethoxybenzaldehdye

-
D
-
34967-25-4
3,5-dimethoxybenzaldoxime

| Conditions | Yield |
|---|---|
| With water In acetonitrile Product distribution; Quantum yield; Further Variations:; Reagents; Solvents; UV-irradiation; | A 74 % Chromat. B 9 % Chromat. C 5 % Chromat. D 37 % Chromat. |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: NaOH 2: SOCl2 3: 92 percent / LiAlH4 / diethyl ether View Scheme | |
| Multi-step reaction with 2 steps 1: potassium carbonate / acetone / 4 h / Reflux 2: lithium aluminium tetrahydride / tetrahydrofuran / 2.17 h / 0 - 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: potassium carbonate / acetone / 4 h / Reflux 2: lithium aluminium tetrahydride / diethyl ether / 1 h / 0 °C View Scheme |

| Conditions | Yield |
|---|---|
| With phosphorus tribromide In benzene at 20℃; | 100% |
| With phosphorus tribromide In 1,4-dioxane at 40℃; for 1h; Inert atmosphere; | 99% |
| Stage #1: 3,5-dimethoxybenzyl alcohol With triphenylphosphine In dichloromethane at 20℃; for 0.166667h; Appel reaction; Stage #2: With carbon tetrabromide In dichloromethane at -78 - -50℃; Appel reaction; | 98% |
-
-
74124-79-1
di(succinimido) carbonate

-
-
705-76-0
3,5-dimethoxybenzyl alcohol

-
-
1383617-02-4
3,5-dimethoxybenzyl-N-succinimidyl carbonate

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 20℃; for 0.333333h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With trichlorophosphate at 0 - 75℃; for 2h; Vilsmeier-Haack Formylation; | 100% |

| Conditions | Yield |
|---|---|
| With silica-supported Jones reagent In dichloromethane for 0.00269444h; | 99.5% |
| With 1,2-dimethyl-3-[6-(methylsulfinyl)hexyl]-1H-imidazolium triflate; oxalyl dichloride; triethylamine In dichloromethane; acetonitrile at -78 - 20℃; Swern oxidation; | 97% |
| With Dess-Martin periodane In dichloromethane at 0℃; Inert atmosphere; | 96% |

| Conditions | Yield |
|---|---|
| With silica-supported Jones reagent In dichloromethane for 0.035h; | 99.3% |
| With C15H27Br2CoN3; potassium hydroxide In toluene at 140℃; for 16h; Cannizzaro Reaction; Inert atmosphere; Sealed tube; | 86% |
| With potassium phosphate; carbon dioxide; CrH6Mo6O24(3-)*3H3N*3H(1+) In dimethyl sulfoxide at 80℃; under 750.075 Torr; for 24h; Green chemistry; | 82% |
-
-
705-76-0
3,5-dimethoxybenzyl alcohol

-
-
74726-77-5
2-iodo-3,5-bis(methoxy)benzenemethanol

| Conditions | Yield |
|---|---|
| With iodine; silver trifluoroacetate | 99% |
| With N-iodo-succinimide In N,N-dimethyl-formamide at 0 - 40℃; for 3h; | 99% |
| With iodine; silver trifluoroacetate In chloroform for 0.25h; | 94% |
-
-
705-76-0
3,5-dimethoxybenzyl alcohol

-
-
108-24-7
acetic anhydride

-
-
38513-65-4
3,5-dimethoxybenzyl acetate

| Conditions | Yield |
|---|---|
| With tin(IV) tetraphenylporphyrin perchlorate at 20℃; for 0.0833333h; | 99% |
| ruthenium trichloride In acetonitrile at 20℃; for 0.416667h; | 95% |
| With pyridine | 93% |
| With pyridine at 20℃; for 3h; Acetylation; | 86% |
| In pyridine |
-
-
705-76-0
3,5-dimethoxybenzyl alcohol

-
-
74726-76-4
2-bromo-3,5-dimethoxybenzyl alcohol

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In tetrachloromethane at 70℃; for 0.666667h; | 98% |
| With N-Bromosuccinimide In chloroform at 45℃; | 96% |
| With N-Bromosuccinimide In dichloromethane at 0 - 25℃; for 12.5h; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; potassium carbonate; benzyl chloride In tetrahydrofuran at 65 - 70℃; for 20h; Inert atmosphere; Schlenk technique; | 98% |
| With bismuth(lll) trifluoromethanesulfonate; dichloro bis(acetonitrile) palladium(II); oxygen; potassium carbonate at 60℃; for 3h; Schlenk technique; | 91% |
| With palladium 10% on activated carbon; oxygen; sodium carbonate at 120℃; under 15001.5 Torr; for 1.5h; Microwave irradiation; Green chemistry; | 36% |

| Conditions | Yield |
|---|---|
| With pyridine; thionyl chloride In diethyl ether at 20℃; Cooling with ice; | 97% |
| With trichlorophosphate In methanol for 2h; Time; Reflux; | 96.7% |
| With trichlorophosphate for 2h; Time; Reflux; | 96.7% |
-
-
705-76-0
3,5-dimethoxybenzyl alcohol

-
-
106-96-7
propargyl bromide

-
-
876366-16-4
1,3-dimethoxy-5-{[(prop-2-yn-1-yl)oxy]methyl}benzene

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran for 10h; | 97% |
-
-
705-76-0
3,5-dimethoxybenzyl alcohol

-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
40243-87-6
3,5-dimethoxystyrene

| Conditions | Yield |
|---|---|
| Stage #1: Methyltriphenylphosphonium bromide With n-butyllithium In tetrahydrofuran; hexane at 0℃; for 1h; Stage #2: 3,5-dimethoxybenzyl alcohol In tetrahydrofuran at 0 - 20℃; for 2h; | 96% |
| Stage #1: Methyltriphenylphosphonium bromide With n-butyllithium In tetrahydrofuran; hexane at 0℃; Wittig type reaction; Stage #2: 3,5-dimethoxybenzyl alcohol With 4,4'-di-tert-butylbiphenyl; lithium; nickel dichloride In tetrahydrofuran; hexane for 8h; Wittig type reaction; Inert atmosphere; Reflux; | 40% |

| Conditions | Yield |
|---|---|
| With pyridine; dmap; 4-methyltetrahydropyran at 20℃; for 0.5h; Inert atmosphere; Green chemistry; | 96% |

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane | 93% |
| With pyridine In benzene Ambient temperature; |
-
-
705-76-0
3,5-dimethoxybenzyl alcohol

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
166322-67-4
2-(chloromethyl)-4,6-dimethoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: N,N-dimethyl-formamide With trichlorophosphate at 25℃; for 0.333333h; Stage #2: 3,5-dimethoxybenzyl alcohol at 25 - 75℃; for 2h; Further stages.; | 93% |
| With trichlorophosphate at 75℃; for 2h; Formylation; substitution; | 93% |
| Stage #1: N,N-dimethyl-formamide With trichlorophosphate at 0 - 20℃; for 0.5h; Vilsmeier-Haack Formylation; Inert atmosphere; Stage #2: 3,5-dimethoxybenzyl alcohol at 0 - 75℃; for 2h; Vilsmeier-Haack Formylation; Inert atmosphere; regioselective reaction; | 93% |
-
-
705-76-0
3,5-dimethoxybenzyl alcohol

-
-
166322-67-4
2-(chloromethyl)-4,6-dimethoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| With trichlorophosphate In N,N-dimethyl-formamide | 93% |
-
-
705-76-0
3,5-dimethoxybenzyl alcohol

-
-
51-44-5
3,4-dichlorbenzoic acid

-
-
1259030-74-4
3,5-dimethoxybenzyl 3,4-dichlorobenzoate

| Conditions | Yield |
|---|---|
| With C18H20N2O4; Nb-TPP In tetrahydrofuran at 0 - 20℃; Mitsunobu reaction; | 93% |
-
-
705-76-0
3,5-dimethoxybenzyl alcohol

-
-
62-53-3
aniline

-
-
1174639-36-1
(E)-N-(3,5-dimethoxybenzylidene)aniline

| Conditions | Yield |
|---|---|
| With CuO*Fe3O4; sodium hydroxide In toluene at 100℃; for 96h; Inert atmosphere; | 93% |

| Conditions | Yield |
|---|---|
| With dmap; triethylamine at 0 - 20℃; for 2h; | 93% |
-
-
705-76-0
3,5-dimethoxybenzyl alcohol

-
-
50827-57-1
2-hydroxymethyl-6-methoxy-1,4-benzoquinone

| Conditions | Yield |
|---|---|
| With C33H33N5O8Ru; dihydrogen peroxide In ethyl acetate at 20℃; for 15h; Schlenk technique; Green chemistry; chemoselective reaction; | 93% |
-
-
705-76-0
3,5-dimethoxybenzyl alcohol

-
-
19179-31-8
3,5-dimethoxy-benzonitrile

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; TEMPOL; ammonia; copper(l) chloride In water; acetonitrile at 20℃; for 24h; | 93% |
| With ammonia; oxygen In 1,4-dioxane for 2h; Reflux; | 90% |
| With ammonia; oxygen In ethanol; water for 2h; Reflux; | 90% |
-
-
705-76-0
3,5-dimethoxybenzyl alcohol

-
-
98-88-4
benzoyl chloride

-
-
138250-67-6
3,5-dimethoxybenzyl benzoate

| Conditions | Yield |
|---|---|
| With pyridine | 92% |
-
-
705-76-0
3,5-dimethoxybenzyl alcohol

-
-
58479-61-1
tert-butylchlorodiphenylsilane

-
-
115130-81-9
(1,1-dimethylethyl)diphenylsilyl 3,5-dimethoxybenzyl ether

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 5h; | 92% |
| With 4-methylpyridine-1-oxide In dichloromethane at 20℃; Molecular sieve; | 92% |
-
-
705-76-0
3,5-dimethoxybenzyl alcohol

-
-
6253-28-7, 19878-26-3, 42074-03-3
exo-7-oxabicyclo[2.2.1]hept-4-ene-2,3-dicarboximide

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 0 - 20℃; for 18h; Inert atmosphere; | 92% |
-
-
705-76-0
3,5-dimethoxybenzyl alcohol

-
-
28144-70-9
anthranilic acid amide

| Conditions | Yield |
|---|---|
| With Iron(III) nitrate nonahydrate; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; oxygen; potassium hydroxide In toluene at 100℃; for 12h; | 92% |

| Conditions | Yield |
|---|---|
| With C30H43ClCoN2P3(1+)*Cl(1-); potassium triethylborohydride; potassium hydroxide In toluene at 140℃; for 24h; Inert atmosphere; Glovebox; Sealed tube; | 92% |
3,5-Dimethoxybenzyl alcohol Specification
The Benzenemethanol,3,5-dimethoxy- with CAS registry number of 705-76-0 is also known as (3,5-Dimethoxyphenyl)methan-1-ol. The IUPAC name is (3,5-Dimethoxyphenyl)methanol. It belongs to product categories of Benzhydrols, Benzyl & Special Alcohols; Alcohols; Anisoles, Alkyloxy Compounds & Phenylacetates; Building Blocks for Dendrimers; Functional Materials; C9 to C30;Oxygen Compounds. Its EINECS registry number is 211-888-6. In addition, the formula is C9H12O3 and the molecular weight is 168.19. This chemical is a white to yellow-beige crystalline solid that insoluble in water. It may cause inflammation to the skin or other mucous membranes that should be sealed in cool, dry place away from oxidants.
Physical properties about Benzenemethanol,3,5-dimethoxy- are: (1)ACD/LogP: 0.75; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.75; (4)ACD/LogD (pH 7.4): 0.75; (5)ACD/BCF (pH 5.5): 2.17; (6)ACD/BCF (pH 7.4): 2.17; (7)ACD/KOC (pH 5.5): 60.62; (8)ACD/KOC (pH 7.4): 60.62; (9)#H bond acceptors: 3; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 4; (12)Index of Refraction: 1.521; (13)Molar Refractivity: 46.06 cm3; (14)Molar Volume: 151.2 cm3; (15)Surface Tension: 37.4 dyne/cm; (16)Density: 1.111 g/cm3; (17)Flash Point: 134.7 °C; (18)Enthalpy of Vaporization: 56.93 kJ/mol; (19)Boiling Point: 299.1 °C at 760 mmHg; (20)Vapour Pressure: 0.000543 mmHg at 25 °C.
Preparation of Benzenemethanol,3,5-dimethoxy-: it is prepared by reaction of 3,5-dimethoxy-benzaldehyde. The reaction needs reagent NaBH4 and solvent methanol at the temperature of 0 °C. The yield is about 93 %.
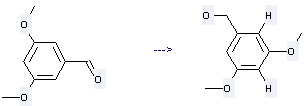
Uses of Benzenemethanol,3,5-dimethoxy-: it is used to produce 2-bromo-3,5-dimethoxy-benzyl alcohol. The reaction occurs with reagent NBS and solvent CHCl3 at the temperature of 45 °C. The yield is about 96 %.

When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes, respiratory system and skin. During using it, wear suitable protective clothing and gloves. Do not breathe dust and avoid contact with skin and eyes. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: COC1=CC(=CC(=C1)CO)OC
2. InChI: InChI=1S/C9H12O3/c1-11-8-3-7(6-10)4-9(5-8)12-2/h3-5,10H,6H2,1-2H3
3. InChIKey: AUDBREYGQOXIFT-UHFFFAOYSA-N
Related Products
- 3,10-Diaminotricyclo(5.2.1.0(sup 2,6))decane
- 3,10-Dinitrophenanthrene
- 3-((10-ETHYL-11-(p-HYDROXYPHENYL)DIBENZ-(B,F)OXEPIN-3-YL)OXY)-1,2-PROPANEDIOL HYDRATE (4:1)
- 3-(1,1,2,2-Tetrafluoroethoxy)aniline
- 3-(1,1,2,2-Tetrafluoroethoxy)benzaldehyde
- 3-(1,1,2,2-Tetrafluoroethoxy)bromobenzene
- 3-(1,1,2,2-Tetrafluoroethoxy)toluene
- 3-[1,1'-Biphenyl]-4-yl-1,2,3,4-tetrahydro-1-naphthol
- 3,11-Dichloro-6,11-dihydro-6-methyldibenzo[c,f][1,2]thiazepine 5,5-dioxide
- 3-[(1,1-Dimethyl-2-hydroxyethyl)amino]-2-hydroxypropanesulfonicacid
- 7057-68-3
- 705-79-3
- 70580-08-4
- 705-84-0
- 70585-60-3
- 7058-59-5
- 70-58-6
- 705-86-2
- 70588-05-5
- 70591-20-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View