-
Name
ACENOCOUMAROL
- EINECS 205-807-3
- CAS No. 152-72-7
- Article Data12
- CAS DataBase
- Density 1.427 g/cm3
- Solubility
- Melting Point 196-199 °C
- Formula C19H15NO6
- Boiling Point 592.718 °C at 760 mmHg
- Molecular Weight 353.331
- Flash Point 312.265 °C
- Transport Information UN 2811
- Appearance White crystalline solid
- Safety 26-36/37
- Risk Codes 63-22-36/37/38
-
Molecular Structure
- Hazard Symbols Xn
- Synonyms Acenocoumarol(6CI);Coumarin, 3-(a-acetonyl-p-nitrobenzyl)-4-hydroxy- (8CI);3-(Alpha-acetonyl-4-nitrobenzyl)-4-hydroxycoumarin;3-(a-Acetonyl-4-nitrobenzyl)-4-hydroxycoumarin;3-(a-Acetonyl-p-nitrobenzyl)-4-hydroxycoumarin;3-(a-p-Nitrophenyl-b-acetylethyl)-4-hydroxycoumarin;3-[a-(4'-Nitrophenyl)-b-acetylethyl]-4-hydroxycoumarin;3-[a-(p-Nitrophenol)-b-acetylethyl]-4-hydroxycoumarin;4-Hydroxy-2-oxo-3-[3-oxo-1-(4-nitrophenyl)butyl]-2H-chromene;Acenocoumarin;Acitrom;Ascumar;DL-3-(a-Acetonyl-4-nitrobenzyl)-4-hydroxycoumarin;G 23,350;Minisintrom;Nicoumalone;Nitrowarfarin;Sinkumar;Sinthrome;Sintrom;Sintrom Mitis;Syncumar;Syntrom;Trombostop;Zotil;
- PSA 113.33000
- LogP 4.04100
Synthetic route
-
-
1076-38-6
4-hydroxy[1]benzopyran-2-one

-
-
946-49-6, 3490-37-7, 30625-98-0
(E)-4-(4-nitrophenyl)-3-buten-2-one

-
-
152-72-7
acenocoumarol

| Conditions | Yield |
|---|---|
| With polystyrene-divinylbenzene support prepared with 2-ethylhexanoic acid as porogen with immobilized 1,5,7-triazabicyclo[4.4.0]dec-5-ene at 100℃; for 72h; Reagent/catalyst; Michael Addition; Green chemistry; | 96% |
| With N-ethyl-N,N-diisopropylamine In water for 18h; Michael Addition; Reflux; | 96% |
-
-
946-49-6, 3490-37-7, 30625-98-0
4-(p-nitrophenyl)-3-butene-2-one

-
-
1076-38-6
4-hydroxy[1]benzopyran-2-one

-
-
152-72-7
acenocoumarol

| Conditions | Yield |
|---|---|
| potassium fluoride; N-benzyl-N,N,N-triethylammonium chloride In water for 2h; Product distribution; Heating; var. catalysts; | 92% |
| potassium fluoride; N-benzyl-N,N,N-triethylammonium chloride In water for 2h; Heating; | 92% |
-
-
1076-38-6
4-hydroxy[1]benzopyran-2-one

-
-
116-11-0
2-Methoxypropene

-
-
555-16-8
4-nitrobenzaldehdye

-
-
152-72-7
acenocoumarol

| Conditions | Yield |
|---|---|
| Stage #1: 4-hydroxy[1]benzopyran-2-one; 2-Methoxypropene; 4-nitrobenzaldehdye With ethylenediamine diacetic acid In 1,4-dioxane at 90℃; tandem Knoevenagel-hetero-Diels-Alder reaction; Stage #2: With hydrogenchloride; silica gel In water; trifluoroacetic acid at 20℃; |

-
-
152-72-7
acenocoumarol

-
-
14602-86-9
(1R,2S,5R)-menthyl chloroformate

| Conditions | Yield |
|---|---|
| With TEA In 1,2-dichloro-ethane for 0.5h; Ambient temperature; | 100% |
-
-
152-72-7
acenocoumarol

| Conditions | Yield |
|---|---|
| With pyridine; sodium hydroxide; hydroxylamine hydrochloride In ethanol 1) 12 h, 2) reflux, 6 h; | 70% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 22h; Reflux; | A 12% B 64% |


| Conditions | Yield |
|---|---|
| for 1h; Heating; | 49.4% |
-
-
152-72-7
acenocoumarol

| Conditions | Yield |
|---|---|
| With hydrogen; nickel |
-
-
152-72-7
acenocoumarol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: H2 / Raney Ni 2: 1.) ice-bath, 1 h, 2.) 0 deg C, overnight View Scheme |
Acenocoumarol Chemical Properties
Chemistry informtion about Acenocoumarol (CAS NO.152-72-7) is:
IUPAC Name: 2-Hydroxy-3-[1-(4-Nitrophenyl)-3-Oxobutyl]Chromen-4-One
Synonyms: 3-(Alpha-(4'-Nitrophenyl)-Beta-Acetylethyl)-4-Hydroxycoumarin ; 3-(Alpha-(P-Nitrophenol)-Beta-Acetylethyl)-4-Hydroxycoumarin ; 3-(Alpha-Acetonyl-4-Nitrobenzyl)-4-Hydroxycoumarin ; 3-(Alpha-Acetonyl-P-Nitrobenzyl)-4-Hydroxy-Coumari ; 3-(Alpha-Acetonyl-P-Nitrobenzyl)-4-Hydroxy-Coumarin ; 3-(Alpha-P-Nitrophenyl-Beta-Acetylethyl)-4-Hydroxycoumarin ; 4-Hydroxy-3-(1-(4-Nitrophenyl)-3-Oxobutyl)-2h-1-Benzopyran-2-On ; 4-Hydroxy-3-(1-(4-Nitrophenyl)-3-Oxobutyl)-2h-1-Benzopyran-2-One
Product Categories: Aromatics ; Chiral Reagents ; Heterocycles ; Intermediates & Fine Chemicals ; Pharmaceuticals
MF: C19H15NO6
MW: 353.33
EINECS: 205-807-3
Density: 1.427 g/cm3
Melting Point: 196-1990°C
Flash Point: 312.3 °C
Boiling Point: 592.7 °C at 760 mmHg
Vapour Pressure: 6.78E-15 mmHg at 25°C
Enthalpy of Vaporization: 92.95 kJ/mol
Following is the molecular structure of Acenocoumarol (CAS NO.152-72-7) is:
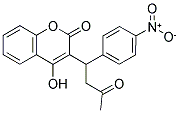
Acenocoumarol Uses
Acenocoumarol (CAS NO.152-72-7) can be used as R-Enantiomer of Acenocoumarol. Vitamin K antagonist; It is structurally similar to Warfarin and used as Anticoagulant.
Acenocoumarol Production
Raw materials are 4-Hydroxycoumarin-->4-Phenyl-1-butene .
Acenocoumarol Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 115mg/kg (115mg/kg) | "Merck Index; an Encyclopedia of Chemicals, Drugs, and Biologicals", 11th ed., Rahway, NJ 07065, Merck & Co., Inc. 1989Vol. 11, Pg. 6, 1989. | |
| mouse | LD50 | oral | 1470mg/kg (1470mg/kg) | Therapie. Vol. 11, Pg. 85, 1956. | |
| rat | LD50 | oral | 513mg/kg (513mg/kg) | "Handbook of Analytical Toxicology," Sunshine, I., ed., Cleveland, OH, Chemical Rubber Co., 1969Vol. -, Pg. 3, 1969. |
Acenocoumarol Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion. A human teratogen by an unspecified route. When heated to decomposition it emits toxic fumes such as NOx. See also WARFARIN.
RIDADR: 2811
HazardClass: 6.1(b)
PackingGroup: III
Acenocoumarol Specification
Acenocoumarol (CAS NO.152-72-7) is a white crystalline solidis.It is an anticoagulant that functions as a vitamin K antagonist (like warfarin). It is a derivative of coumarin and is marketed under the brand names Sintrom and Sinthrome.
Related Products
- Acenocoumarol
- 1527-34-0
- 152735-23-4
- 15274-43-8
- 152751-57-0
- 152763-53-6
- 15277-97-1
- 152787-66-1
- 1527-89-5
- 15278-97-4
- 1527-91-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View