-
Name
artemisic acid
- EINECS
- CAS No. 80286-58-4
- Article Data5
- CAS DataBase
- Density 1.019 g/cm3
- Solubility
- Melting Point 129-131 °C
- Formula C15H22O2
- Boiling Point 373.563 °C at 760 mmHg
- Molecular Weight 234.338
- Flash Point 273.336 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Arteannuic acid;Artemisininic acid;Qing Hao acid;
- PSA 37.30000
- LogP 3.64580
Synthetic route
-
-
125184-95-4
dehydroartemisinyl alcohol

-
-
80286-58-4
artemisinic acid

| Conditions | Yield |
|---|---|
| With Jones reagent In acetone at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| With Tetradecanoic acid 1-methylethyl ester; artemisinic acid producing yeast strain In water for 123.4h; Product distribution / selectivity; Enzymatic reaction; |
-
-
64-17-5
ethanol

-
B
-
80286-58-4
artemisinic acid

-
C
-
92692-39-2
(1R,4R,4aS,8aR)-4,7-dimethyl-1-(prop-1-en-2-yl)-1,2,3,4,4a,5,6,8a-octahydronaphthalene

-
D
-
125184-95-4
dehydroartemisinyl alcohol

| Conditions | Yield |
|---|---|
| With Tetradecanoic acid 1-methylethyl ester Reagent/catalyst; Enzymatic reaction; |

-
-
64-17-5
ethanol

-
A
-
80286-58-4
artemisinic acid

-
B
-
92692-39-2
(1R,4R,4aS,8aR)-4,7-dimethyl-1-(prop-1-en-2-yl)-1,2,3,4,4a,5,6,8a-octahydronaphthalene

-
C
-
125184-95-4
dehydroartemisinyl alcohol

| Conditions | Yield |
|---|---|
| With Tetradecanoic acid 1-methylethyl ester Reagent/catalyst; Enzymatic reaction; |


| Conditions | Yield |
|---|---|
| In diethyl ether at 0℃; | 100% |
| In diethyl ether at 0℃; | 99% |
| In diethyl ether for 0.333333h; Ambient temperature; | 98% |
-
-
80286-58-4
artemisinic acid

-
-
85031-59-0, 110715-68-9, 126643-10-5
dihydroartemisinic acid

| Conditions | Yield |
|---|---|
| With lithium borohydride; nickel dichloride In methanol for 2h; Ambient temperature; | 100% |
| With C48H50Cl4N2O2P2Ru3; hydrogen; sodium hydrogencarbonate In methanol at 0℃; under 22502.3 Torr; for 36h; Autoclave; | 98% |
| With palladium on activated charcoal; hydrogen In chloroform at 20℃; under 750.075 Torr; Solvent; Pressure; Reagent/catalyst; | 98% |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
80286-58-4
artemisinic acid

-
-
150665-64-8
(S)-3-((1R,4R,4aS,8aR)-4,7-Dimethyl-1,2,3,4,4a,5,6,8a-octahydro-naphthalen-1-yl)-4,5-dihydro-3H-pyrazole-3-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| In diethyl ether at 0℃; for 10h; | 98% |
-
-
80286-58-4
artemisinic acid

-
-
110715-68-9
(S)-dihydroartemisinic acid

| Conditions | Yield |
|---|---|
| With C48H50Cl4N2O2P2Ru3; hydrogen; sodium hydrogencarbonate In methanol at 0℃; under 22502.3 Torr; for 36h; Autoclave; | 98% |
| With dihydrogen peroxide; hydrazine hydrate In ethanol at 0 - 20℃; | 86% |
-
-
80286-58-4
artemisinic acid

-
-
161022-41-9
dihydroartemisinic acid

| Conditions | Yield |
|---|---|
| With hydrogen; potassium hydroxide In water; toluene at 0 - 60℃; under 76005.1 Torr; for 12h; Reagent/catalyst; Solvent; Autoclave; enantioselective reaction; | 97.4% |
| With hydrogen; Wilkinson's catalyst In methanol under 1551.49 - 1913.5 Torr; for 289h; Product distribution / selectivity; Parr apparatus; | |
| With tris(triphenylphosphine)ruthenium(II) chloride; C48H50N2O2P2Ru; hydrogen; triethylamine In methanol at 0℃; under 15001.5 Torr; for 24h; Autoclave; enantioselective reaction; | n/a |
-
-
80286-58-4
artemisinic acid

-
-
2450-71-7
Propargylamine

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 25℃; for 6h; Inert atmosphere; | 95% |
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 25℃; for 6h; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 1h; Heating; | 94% |
-
-
80286-58-4
artemisinic acid

-
-
107-19-7
propargyl alcohol

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 25℃; for 12h; | 94% |
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 25℃; for 12h; | 94% |
-
-
17376-04-4
2-phenethyl iodide

-
-
80286-58-4
artemisinic acid

| Conditions | Yield |
|---|---|
| With triethyl borane; tri-n-butyl-tin hydride In hexane at 20℃; for 16h; Addition; | 72% |
-
-
80286-58-4
artemisinic acid

-
-
125184-95-4
dehydroartemisinyl alcohol

| Conditions | Yield |
|---|---|
| Stage #1: artemisinic acid With lithium aluminium tetrahydride In tetrahydrofuran at 70℃; for 15h; Heating / reflux; Stage #2: With sodium hydroxide; water In tetrahydrofuran for 0.166667h; | 65% |
| With diisobutylaluminium hydride | |
| Multi-step reaction with 2 steps 1: 99 percent / diethyl ether / 0 °C 2: 82 percent / DIBAL / CH2Cl2 / -78 °C View Scheme |
-
-
80286-58-4
artemisinic acid

-
A
-
118059-19-1
3-β-hydroxyartemisinic acid

| Conditions | Yield |
|---|---|
| With endophytic fungus Trichothecium roseum CIMAPN1 In aq. phosphate buffer; acetone at 28℃; for 336h; pH=6; Microbiological reaction; stereoselective reaction; | A 51.1% B 37.3% |
-
-
80286-58-4
artemisinic acid

| Conditions | Yield |
|---|---|
| With cell culture of endophytic fungus Trichothecium roseum In acetone for 336h; Microbiological reaction; | A 50.4% B 32.2% C 5.2% |


| Conditions | Yield |
|---|---|
| With whole-cell of E. coli BL21 culture at 25℃; for 72h; pH=7.0; aq. buffer; Enzymatic reaction; | 18.6% |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
80286-58-4
artemisinic acid

-
-
129165-35-1
methyl <2'R,4a'S,5'R,8'R>-2-(2',5'-dimethyl-2'-hydroperoxy-2',3',4',4a',5',6',7',8'-octahydronaphthalen-8'-yl)propenoate

| Conditions | Yield |
|---|---|
| With oxygen; rose bengal 1.) acetonitrile, -30 deg C, irradiation, 2.) ether, 0 deg C; Yield given. Multistep reaction; |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
80286-58-4
artemisinic acid

-
A
-
87391-99-9
2-(4,7-dimethyl-(1α-H),2,3,(4β-H),(4aα-H),5,6,(8aα-H)-octahydronaphthalen-1-yl)propionic acid methyl ester

-
B
-
85788-54-1, 87391-99-9, 98575-64-5
11S-methyl arteannuinate

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; nickel dichloride 1.) MeOH, -15 deg C, 2.) 30 min; Multistep reaction; |

-
-
80286-58-4
artemisinic acid

-
-
101020-89-7
dehydroqinghaosu

| Conditions | Yield |
|---|---|
| With oxygen; copper(II) bis(trifluoromethanesulfonate); rose bengal 1.) acetonitrile, -30 deg C, 2.) from -20 deg C to RT, 12 h; Yield given. Multistep reaction; |
-
-
80286-58-4
artemisinic acid

-
A
-
63968-64-9
C12H13O2(CH3)3(O)(OO)

-
B
-
50906-56-4
(1R,5S,8R,9S,12R,14R)-8,12-dimethyl-4-methylidene-2,13-dioxatetracyclo[7.5.0.01,5.012,14]tetradecan-3-one

| Conditions | Yield |
|---|---|
| In water at 30℃; for 2h; cell-free extract from Artemisia annua L. (Asteraceae), EDTA, HEPES with DDT buffer, pH 7.15; Title compound not separated from byproducts; |
-
-
80286-58-4
artemisinic acid

| Conditions | Yield |
|---|---|
| With oxygen; methylene blue In ethanol at 22℃; for 4h; Irradiation; Yield given; |
-
-
80286-58-4
artemisinic acid

| Conditions | Yield |
|---|---|
| microbial fermentation by Mucor mucedo; Yield given; |
-
-
80286-58-4
artemisinic acid


| Conditions | Yield |
|---|---|
| microbial fermentation by Aspergilus flavipes; var. organism; Yield given. Yields of byproduct given; |
-
-
80286-58-4
artemisinic acid

-
-
128261-36-9
2-((1R,4R,4aS,7R)-7-Hydroxy-4,7-dimethyl-1,2,3,4,4a,5,6,7-octahydro-naphthalen-1-yl)-acrylic acid

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; oxygen; triethyl phosphite; methylene blue 1.) irradiation, CH2Cl2, -78 deg C, 2.5 h; 2.) Et2O-petrol, rt, 30 min; 3.) t-BuOH; Yield given. Multistep reaction; |
-
-
80286-58-4
artemisinic acid

-
A
-
128261-36-9
2-((1R,4R,4aS,7R)-7-Hydroxy-4,7-dimethyl-1,2,3,4,4a,5,6,7-octahydro-naphthalen-1-yl)-acrylic acid

| Conditions | Yield |
|---|---|
| With oxygen; sodium carbonate; triphenylphosphine; methylene blue 1.) irradiation, CH2Cl2, -78 deg C, 2.5 h; 2.) to -15 deg C, 30 min; 3.) water, 100 deg C, 2h; Yield given. Multistep reaction. Yields of byproduct given; |
-
-
80286-58-4
artemisinic acid

| Conditions | Yield |
|---|---|
| With oxygen; methylene blue In dichloromethane at -78℃; for 2.5h; Irradiation; |

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran at 0℃; for 0.25h; | |
| With n-butyllithium 1) THF, 0 deg C, 15 min, 2) THF, 0 deg C, 15 min; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran at 0℃; for 0.25h; | |
| With n-butyllithium 1) THF, 0 deg C, 15 min, 2) THF, 0 deg C, 15 min; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran at 0℃; for 0.25h; | |
| With n-butyllithium 1) THF, 0 deg C, 15 min, 2) THF, 0 deg C, 15 min; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran at 0℃; for 0.25h; |

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran at 0℃; for 0.25h; |
Artemisinic acid Specification
The Artemisinic acid, with the CAS registry number 80286-58-4, is also known as Arteannuic acid. It belongs to the product category of Miscellaneous Natural Products. This chemical's molecular formula is C15H22O2 and molecular weight is 234.34. What's more, both its IUPAC name and systematic name are the same which is called 2-[(1R,4R,4aS,8aR)-4,7-Dimethyl-1,2,3,4,4a,5,6,8a-octahydronaphthalen-1-yl]prop-2-enoic acid.
Physical properties about Artemisinic acid are: (1)ACD/LogP: 5.11; (2)# of Rule of 5 Violations: 1; (3)ACD/LogD (pH 5.5): 3.91; (4)ACD/LogD (pH 7.4): 2.12; (5)ACD/BCF (pH 5.5): 281.08; (6)ACD/BCF (pH 7.4): 4.56; (7)ACD/KOC (pH 5.5): 895.70; (8)ACD/KOC (pH 7.4): 14.53; (9)#H bond acceptors: 2; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 37.3 Å2; (13)Index of Refraction: 1.505; (14)Molar Refractivity: 68.127 cm3; (15)Molar Volume: 229.854 cm3; (16)Polarizability: 27.008×10-24cm3; (17)Surface Tension: 34.031 dyne/cm; (18)Density: 1.019 g/cm3; (19)Flash Point: 273.336 °C; (20)Enthalpy of Vaporization: 68.177 kJ/mol; (21)Boiling Point: 373.563 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25 °C.
Uses of Artemisinic acid: it is used to produce other chemicals. For example, it can react with qinghao acid to get methyl artemisinate. This reaction needs solvent diethyl ether at temperature of 0 °C. The reaction time is 30 min. The yield is 98 %.
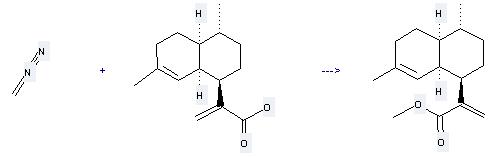
You can still convert the following datas into molecular structure:
(1) SMILES: OC(=O)C(=C)[C@@H]1CC[C@@H](C)[C@@H]2CCC(\C)=C/[C@H]12
(2) InChI: InChI=1S/C15H22O2/c1-9-4-6-12-10(2)5-7-13(14(12)8-9)11(3)15(16)17/h8,10,12-14H,3-7H2,1-2H3,(H,16,17)/t10-,12+,13+,14+/m1/s1
(3) InChIKey: PLQMEXSCSAIXGB-SAXRGWBVSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View