-
Name
Bromocriptine
- EINECS 247-128-5
- CAS No. 25614-03-3
- Article Data9
- CAS DataBase
- Density 1.52 g/cm3
- Solubility 2.07mg/L(temperature not stated)
- Melting Point 215-218° (dec)
- Formula C32H40 Br N5 O5
- Boiling Point 891.3 °C at 760 mmHg
- Molecular Weight 654.604
- Flash Point 492.8 °C
- Transport Information
- Appearance white crystalline powder
- Safety Poison by intravenous and possibly other routes. Human teratogenic effects by an unspecified route: developmental abnormalities of the respiratory system, musculoskeletal system, urogenital system, craniofacial area and body wall. Human systemic effects by ingestion including: olfaction changes. An experimental teratogen. Other experimental reproductive effects. Human mutation data reported. Questionable carcinogen with experimental tumorigenic data. When heated to decomposition it emits very toxic fumes such as Br− and NOx.
- Risk Codes R20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, Xi
Xi
- Synonyms Ergocryptine,2-bromo- (8CI); 8H-Oxazolo[3,2-a]pyrrolo[2,1-c]pyrazine,ergotaman-3',6',18-trione deriv.; Indolo[4,3-fg]quinoline,ergotaman-3',6',18-trione deriv.; (+)-Bromocriptine; 2-Bromo-a-ergocryptine; 2-Bromo-a-ergokryptine;2-Bromoergocriptine; 2-Bromoergocryptine; Bromergocryptine; Bromocriptin;Bromocriptine; Bromocryptine; Bromoergocryptine; Ergolactin; SAN 15-754; Sandoz15-754; a-Bromocryptine; a-Bromoergocryptine; a-Ergolactin
- PSA 118.21000
- LogP 3.39740
Synthetic route
-
-
511-09-1
α-ergocryptine

-
-
25614-03-3
Bromocriptine

| Conditions | Yield |
|---|---|
| With bromodimethylsulfoxonium bromide In dimethyl sulfoxide for 0.166667h; Ambient temperature; | 93% |
| With pyridazinium bromide; 3-bromo-6-chloro-2-methylimidazo In dichloromethane for 0.0333333h; Ambient temperature; | 75% |
| With ammonium hydroxide; sodium hydroxide In dichloromethane; di-isopropyl ether; water; hydrogen bromide; dimethyl sulfoxide |

| Conditions | Yield |
|---|---|
| With water; hydrogen bromide In dimethyl sulfoxide for 0.333333h; Product distribution; Ambient temperature; halogenation of indole alkaloids with various halogenated agents; | A 8% B 72% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; sodium chloride In ice-water; dichloromethane; di-isopropyl ether; dimethyl sulfoxide; ethyl acetate |

| Conditions | Yield |
|---|---|
| 4.7 g (0.007243 mole, 85%) | |
| 4.7 g (0.007243 mole, 85%) |


| Conditions | Yield |
|---|---|
| With ammonium hydroxide; sodium chloride In ice-water; dimethyl sulfoxide; ethyl acetate | mole, 87%) |
| With ammonium hydroxide; sodium chloride In ice-water; dimethyl sulfoxide; ethyl acetate | 6.3 g (0.00967 mole, 87%) |


| Conditions | Yield |
|---|---|
| 0.9 g (0.001359 mole, 91.5%) | |
| 0.9 g (0.001359 mole, 91.5%) |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; sodium chloride In ice-water; dichloromethane; dimethyl sulfoxide; ethyl acetate |

-
-
25614-03-3
Bromocriptine

| Conditions | Yield |
|---|---|
| In butanone |
-
-
3081-61-6, 5822-62-8, 17010-37-6, 34271-54-0
N-ethyl-L-glutamine

-
-
25614-03-3
Bromocriptine

| Conditions | Yield |
|---|---|
| In water; isopropyl alcohol |

| Conditions | Yield |
|---|---|
| In methanol; water at 4℃; for 2.58333h; Solvent; |
Bromocriptine Chemical Properties
Molecular Formula: C32H40BrN5O5
Molar mass: 654.59 g/mol
EINECS: 247-128-5
Density: 1.23 g/cm3
Flash Point: 201.2 °C
Index of Refraction: 1.562
Boiling Point: 608.7 °C at 760 mmHg
Vapour Pressure: 2.34E-17 mmHg at 25°C
Appearance: A white crystalline powder
Product categories of Bromocriptine (25614-03-3): Organics
Structure of Bromocriptine (25614-03-3):
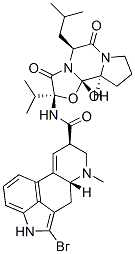
XLogP3-AA: 3.8
H-Bond Donor: 3
H-Bond Acceptor: 6
SMILES: Brc2nc1cccc\3c1c2C[C@@H]7C/3=C/[C@@H](C(=O)N[C@@]4(O[C@]6(O)N(C4=O)[C@H](C(=O)N5CCC[C@H]56)CC(C)C)C(C)C)CN7C
InChI: InChI=1/C32H40BrN5O5/c1-16(2)12-24-29(40)37-11-7-10-25(37)32(42)38(24)30(41)31(43-32,17(3)4)35-28(39)18-13-20-19-8-6-9-22-26(19)21(27(33)34-22)14-23(20)36(5)15-18/h6,8-9,13,16-18,23-25,34,42H,7,10-12,14-15H2,1-5H3,(H,35,39)/t18-,23-,24+,25+,31-,32+/m1/s1
InChIKey: OZVBMTJYIDMWIL-AYFBDAFIBH
Std. InChI: InChI=1S/C32H40BrN5O5/c1-16(2)12-24-29(40)37-11-7-10-25(37)32(42)38(24)30(41)31(43-32,17(3)4)35-28(39)18-13-20-19-8-6-9-22-26(19)21(27(33)34-22)14-23(20)36(5)15-18/h6,8-9,13,16-18,23-25,34,42H,7,10-12,14-15H2,1-5H3,(H,35,39)/t18-,23-,24+,25+,31-,32+/m1/s1
Std. InChIKey: OZVBMTJYIDMWIL-AYFBDAFISA-N
Bromocriptine Uses
Bromocriptine (25614-03-3) is an ergoline derivative and is a dopamine agonist that is used in the treatment of Parkinson's disease (PD), pituitary tumors, and neuroleptic malignant syndrome.Galactorrhea, female infertility, amenorrhea, hypogonadism, and acromegaly (caused by pituitary problems, such as hyperprolactinaemia) may be treated by this drug.
Bromocriptine Production
Bromocriptine (25614-03-3) can be obtained by the reaction of Ergocryptine and N-bromo-succinic acid imide .
Bromocriptine Toxicity Data With Reference
| 1. | oms-hmn:lym 100 µmol/L | MUREAV Mutation Research. 117 (1983),163. | ||
| 2. | dna-rat-ipr 4 mg/kg | CNREA8 Cancer Research. 36 (1976),2223. | ||
| 3. | orl-rat TDLo:7 g/kg/2Y-C:ETA,REP | BMJOAE British Medical Journal. 2 (1977),1605. | ||
| 4. | orl-wmn TDLo:6 mg/kg/60D-I:NOSE | NEJMAG New England Journal of Medicine. 306 (1982),178. | ||
| 5. | ivn-rat LD50:72 mg/kg | DRUGAY Drugs. International Journal of Current Therapeutics and Applied Pharmacology Reviews. 17 (1978),313. | ||
| 6. | ivn-rbt LD50:12 mg/kg | USXXAM United States Patent Document. (Commissioner of Patents and Trademarks, Washington, DC 20231) #3752814 . | ||
| 7. | unr-mus LD50:200 mg/kg | BBIADT Biomedica Biochimica Acta. 43 (1984),1305. |
Bromocriptine Consensus Reports
EPA Genetic Toxicology Program.
Bromocriptine Safety Profile
Poison by intravenous and possibly other routes. Human teratogenic effects by an unspecified route: developmental abnormalities of the respiratory system, musculoskeletal system, urogenital system, craniofacial area and body wall. Human systemic effects by ingestion including: olfaction changes. An experimental teratogen. Other experimental reproductive effects. Human mutation data reported. Questionable carcinogen with experimental tumorigenic data. When heated to decomposition it emits very toxic fumes such as Br− and NOx.
Bromocriptine Specification
Bromocriptine (25614-03-3) also can be called Parlodel ; Bromocriptin ; 2-Bromoergocryptine ; (5'a)-2-Bromo-12'-hydroxy-2'-(-1-methylethyl)-5'-(2-methylpropyl)ergotaman-3',6',18-trione ; Ergotaman-3',6',18-trione, 2-bromo-12'-hydroxy-2'-(1-methylethyl)-5'alpha-(2-methylpropyl)- and Bromocryptin .Its use has been associated with causing or worsening psychotic symptoms: its mechanism is in opposition of most antipsychotics, whose mechanisms generally block dopamine.
Related Products
- Bromocriptine
- Bromocriptine mesylate
- 25617-97-4
- 25618-55-7
- 25619-56-1
- 25620-78-4
- 256224-13-2
- 256228-64-5
- 256228-69-0
- 2562-37-0
- 25625-62-1
- 25628-75-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View