 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
-
Name
Calcium peroxide
- EINECS 215-139-4
- CAS No. 1305-79-9
- Density 2.92
- Solubility insoluble in water, dissolve in acid to form hydrogen peroxide
- Melting Point >365 °C(lit.)
- Formula CaO2
- Boiling Point 150.2 °C at 760 mmHg
- Molecular Weight 72.08
- Flash Point
- Transport Information UN 1457 5.1/PG 2
- Appearance yellowish powder
- Safety 17-26-36/37/39
- Risk Codes 9-36/37/38-34
-
Molecular Structure
-
Hazard Symbols
 O,
O, Xi,
Xi,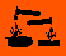 C
C
- Synonyms Calper;Calper G;Neocaloxo;Calciumdioxide;Calcium oxide (CaO2);Calcium peroxide(Ca(O2));
- PSA 46.12000
- LogP -0.23760
Calcium peroxide Consensus Reports
Calcium peroxide Standards and Recommendations
Calcium peroxide Specification
The Calcium peroxide, also known as Calper G, is an organic compound with the formula CaO2. It belongs to the product category of Inorganics. Its EINECS registry number is 215-139-4. With the CAS registry number 1305-79-9, its systematic name is calcium dioxidanediide.
Physical properties of Calcium peroxide: (1)ACD/LogP: -0.43; (2)ACD/LogD (pH 5.5): -0.42; (3)ACD/LogD (pH 7.4): -0.42; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)#H bond acceptors: 2; (7)#H bond donors: 2; (8)#Freely Rotating Bonds: 1; (9)Enthalpy of Vaporization: 45.09 kJ/mol; (10)Boiling Point: 150.2 °C at 760 mmHg; (11)Vapour Pressure: 1.48 mmHg at 25°C.
Preparation: this chemical can be prepared by calcium chloride, hydrogen peroxide and ammonia water. The reaction mixture is stirred at -3~+2 °C with centrifugal separation. Then you can gain product by dry dehydration in 150 ~ 200 °C.
CaCl2 + H2O2+ 2NH3 + 8H2O → CaO2.8H2O + 2NH4Cl
CaO2.H2O → CaO2 + 8H2O
Uses: Calcium peroxide is manufactured to varying specifications and purity and can be used in different areas of industry and agriculture. In agriculture it is used as an oxygen fertilizer, and is also used in the presowing treatments of rice seed. Also, calcium peroxide has found use in the aquaculture industry as it is used to oxygenate and disinfect water, and in the ecological restoration industry as it is used in the treatment of soils. Calcium Peroxide is used in a similar manner to magnesium peroxide for environmental restoration programs. It is used to restore soil and groundwater contaminated with petroleum hydrocarbons by stimulating aerobic microbial degradation of the contaminants in a process known as Enhanced In-Situ Bioremediation.
When you are using this chemical, please be cautious about it as the following:
This chemical may cause inflammation to the skin or other mucous membranes. It is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. Whenever you will contact it, please wear suitable protective clothing, gloves and eye/face protection.
You can still convert the following datas into molecular structure:
(1)SMILES: [Ca+2].[O-][O-]
(2)InChI: InChI=1/Ca.O2/c;1-2/q+2;-2
(3)InChIKey: LHJQIRIGXXHNLA-UHFFFAOYAW
Related Products
- Calcium
- Calcium valproate
- Calcium (1)-bis(2-hydroxy-4-methylvalerate)
- Calcium (2S)-2-[(4-chlorobenzoyl)amino]-3-(1H-indol-3-yl)propanoate
- Calcium (S)-3-methyl-2-oxovalerate
- Calcium 2,4-dichlorophenolate
- Calcium 2-ethylhexanoate
- Calcium 2-hydroxypropanoate
- Calcium 2-methoxyethoxide
- Calcium 2-oxoglutarate
- 13058-04-3
- 13058-16-7
- 13058-24-7
- 130591-93-4
- 13059-60-4
- 130597-31-8
- 130600-36-1
- 13060-24-7
- 130606-48-3
- 1306-06-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View