Synthetic route
-
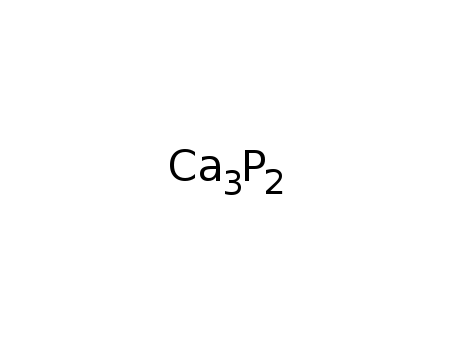
-
calcium phosphide
-
A

-
VP0.95
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) heating at 1200°C;; | A 100%
B n/a |
-
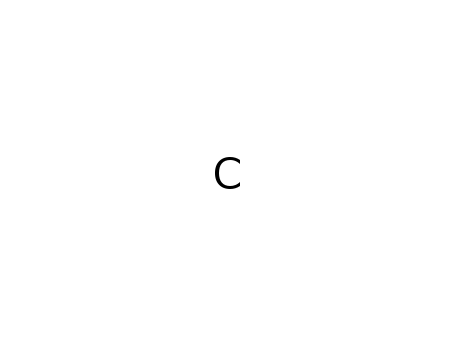
-
graphite
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) pressure about 1 Torr, 1600-1800°C; small amts. of Ca;; | A 94%
B n/a |
| In neat (no solvent) pressure about 1 Torr, 1600-1800°C; small amts. of Ca;; | A 94%
B n/a |
-
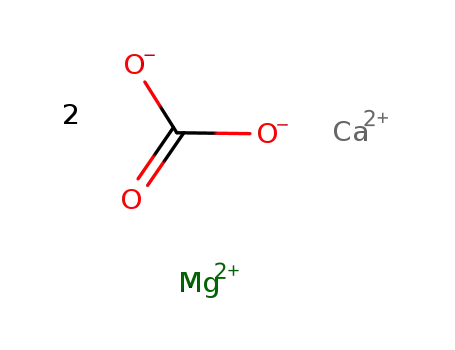
-
dolomite
Conditions
| Conditions | Yield |
|---|
| With pyrographite In melt byproducts: S; Electrolysis; Bitterfeld process variants;; | |
-
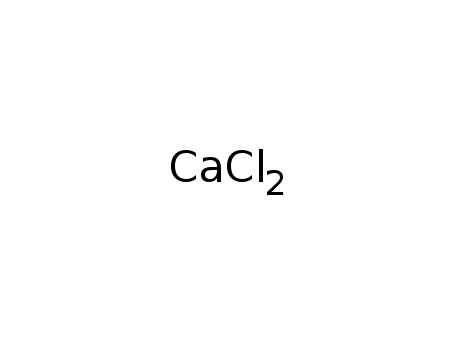
-
calcium chloride
Conditions
| Conditions | Yield |
|---|
| With H In neat (no solvent) byproducts: HCl, H2O; bombarding mixt. of CaCl2:Al2O3=1:4 (on substrate, periodically stirred) with H for 300 mins in plasmochemical app. described by I. Sh. Normatov, N. Shermatov, and U. Mirsaidov, Fiz. Khim. Obrab. Mater., No. 3, 141-142 (1990); mechanism discussed;; X-ray diffraction;; | |
-
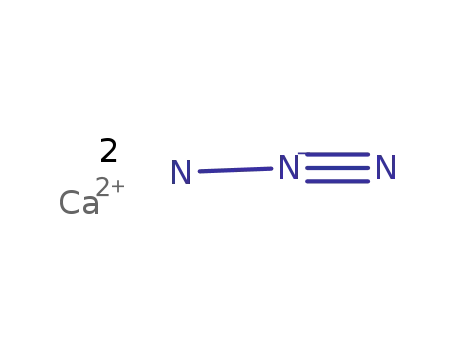
-
calcium azide
Conditions
| Conditions | Yield |
|---|
| explosive decompn. by heating in vacuo at 158°C; | |
-
-
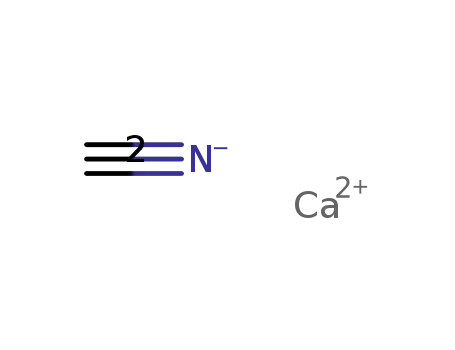
calcium cyanide
Conditions
| Conditions | Yield |
|---|
| In melt Electrolysis; Bitterfeld process variants: at 700 - 800°C;; | |
| In melt Electrolysis; Bitterfeld process variants: at 700 - 800°C;; | |
-

-
calcium cyanide
Conditions
| Conditions | Yield |
|---|
| In melt Electrolysis; Bitterfeld process variants: separated cathode- and anode room; carbon anode and Ni cathode; 2.5 A, 9 V;; | |
| In melt Electrolysis; Bitterfeld process variants: separated cathode- and anode room; carbon anode and Ni cathode; 2.5 A, 9 V;; | |
-

-
calcium(II) sulfide
-
-
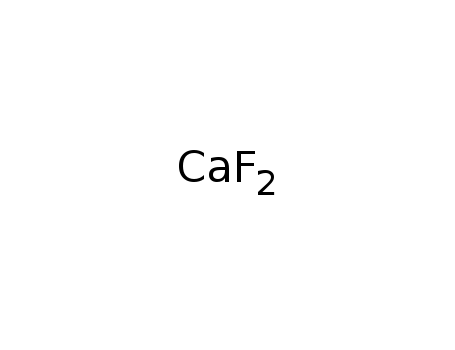
calcium fluoride
-

-
calcium(II) sulfide
Conditions
| Conditions | Yield |
|---|
| In melt Electrolysis; Bitterfeld process variants: under H2 or Ar at ambient or low pressure;; | |
-

-
calcium(II) sulfide
Conditions
| Conditions | Yield |
|---|
| In not given reduction;; | |
-

-
calcium oxide
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) at 1280°C;; | |
| With Al-Si-Fe-Ti alloy In neat (no solvent) reduction by alloy;; | |
| With aluminium In neat (no solvent) Pidgeon process: at 1200°C in vacuum;; | |
-

-
calcium oxide
Conditions
| Conditions | Yield |
|---|
| incomplete reaction {4,6,14,16,17} with formation of yellow powder with bad odor {14};; | |
-
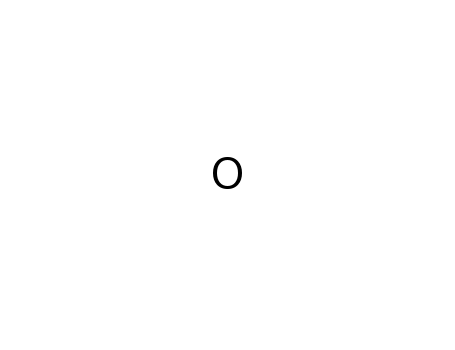
-
oxygen
-

-
calcium oxide
Conditions
| Conditions | Yield |
|---|
| In gaseous matrix Kinetics; byproducts: O2; He bath gas, 250-898 K; time-resolved laser-indiced fluorescence spectroscopic monitoring; | |
-

-
calcium oxide
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) react. in the heat;; | |
-

-
calcium oxide
-
A
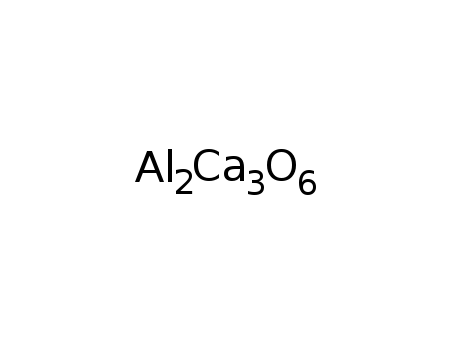
-
tricalcium aliminate
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) reduction with aluminium;; | |
| In neat (no solvent) reduction with aluminium;; | |
-

-
calcium chloride
-

-
calcium oxide
Conditions
| Conditions | Yield |
|---|
| In melt byproducts: O2, CO2, Cl2; Electrolysis; electrowinning of Ca metal from molten CaCl2 containing 0-5 wt% CaO at 825-900°C in MgO crucible, under Ar atm., stainless steel cathode and graphite anode (K and Na chlorides for lowering melting point, density and viscosity); anodically generated gaseous by-products O2, CO2 and Cl2 removed; | |
-

-
calcium carbonate
-

-
calcium chloride
-

-
calcium oxide
Conditions
| Conditions | Yield |
|---|
| With pyrographite In melt Electrolysis; Bitterfeld process variants: cyclic process;; | |
| With pyrographite In melt Electrolysis; Bitterfeld process variants: cyclic process;; | |
-

-
calcium oxide
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) reduction of CaO with NaCN at 560-750°C in vac.;; no complete reaction;; | |
| In neat (no solvent) reduction of CaO with NaCN at 560-750°C in vac.;; no complete reaction;; | |
-

-
calcium fluoride
-

-
calcium oxide
Conditions
| Conditions | Yield |
|---|
| With aluminium In neat (no solvent) reduction in vacuum;; | |
| With silicon In neat (no solvent) reduction in vacuum;; | |
| With Al In neat (no solvent) reduction in vacuum;; | |
-

-
calcium oxide
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) | |
| In not given reduction;; | |
| In not given reduction;; | |
-
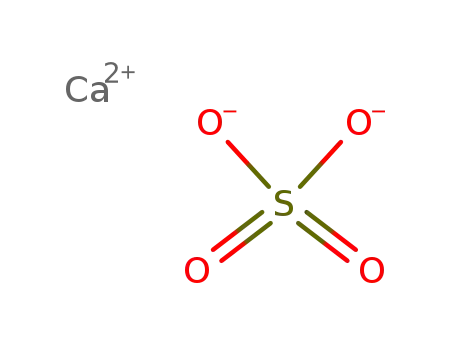
-
calcium sulfate
-

-
calcium oxide
Conditions
| Conditions | Yield |
|---|
| With S In neat (no solvent) heating on between mp and bp of Ca; thermal decompn.;; | |
| With sulfur In neat (no solvent) heating on between mp and bp of Ca; thermal decompn.;; | |
-

-
calcium carbonate
-

-
calcium oxide
Conditions
| Conditions | Yield |
|---|
| With S In neat (no solvent) heating on between mp and bp of Ca; thermal decompn.;; | |
| With sulfur In neat (no solvent) heating on between mp and bp of Ca; thermal decompn.;; | |
-

-
calcium oxide
-

-
calcium chloride
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) shaking of Na, CaCl2 and NaCl in a closed supremax-tube for 30 min at 500-810°C until equilibrium; heating for 15 min at the same temperature; chilling; examination of the equilibrium;; seperation of the saltfree metal- and the saltphase under ether;; | |
-
A

-
arsenic
-
B

-
tetrafluoroboric acid
-
C
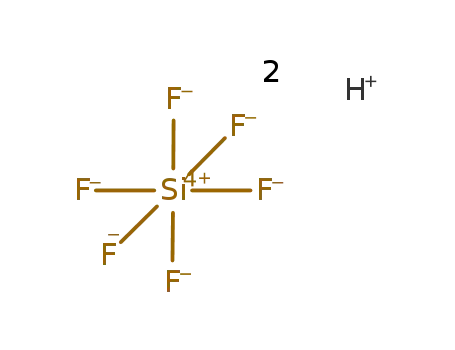
-
fluorosilicic acid
Conditions
| Conditions | Yield |
|---|
| With hydrogen fluoride byproducts: Na, H2SO4; with ice cooling; H2SO4 and HF removed by Pb(CO3)2, Pb by H2S and H2S by air stream; | |
| With HF byproducts: Na, H2SO4; with ice cooling; H2SO4 and HF removed by Pb(CO3)2, Pb by H2S and H2S by air stream; | |
Conditions
| Conditions | Yield |
|---|
| With calcium oxide | |
| With calcium silicate | |
Conditions
| Conditions | Yield |
|---|
| With calcium oxide treatment by heating in vac.; after cooling treatment with melted Na and alcohol; | |
| With calcium oxide | 0% |
| With hydrogen; calcium oxide at red heat in H2 flow; | 0% |
| With CaO treatment by heating in vac.; after cooling treatment with melted Na and alcohol; | |
-

-
calcium chloride
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) equilibrium reaction at higher temperatures;; | |
Conditions
| Conditions | Yield |
|---|
| With calcium oxide at red heat; 8.3 % Ca in the product; | |
-

-
Ca6PtCd11
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) at 600 - 950℃; for 111h; Sealed tube; Schlenk technique; Inert atmosphere; Glovebox; | 100% |
Conditions
| Conditions | Yield |
|---|
| In melt High Pressure; Ca melted in vac. at 350°C for 5 h, filled with H2 (5 atm) at 400°C for 5 min, at 10 atm. at 480°; elem. anal.; | 99.6% |
| In neat (no solvent) hydrogenation in a closed iron tube under pressure at temperatures between 650°C and 690°C;; | 90% |
| In neat (no solvent) hydrogenation in a closed iron tube under pressure at temperatures between 650°C and 690°C;; | 90% |
-
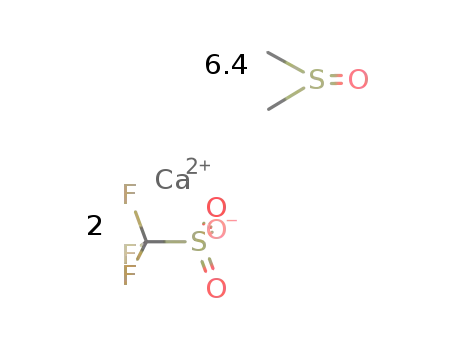
-
calcium(II) triflate - dimethylsulfoxide (1/6.4)
Conditions
| Conditions | Yield |
|---|
| With oxygen In dimethyl sulfoxide metal. Ca under O2 atm. treated with DMSO and triflic acid (2 equiv.) in3 portions, heated at 100°C for 2 h; | 99% |
-
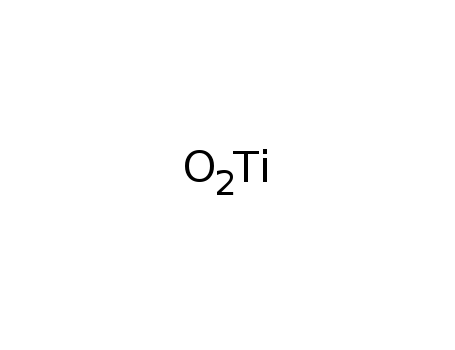
-
titanium(IV) oxide
-
B

-
calcium oxide
Conditions
| Conditions | Yield |
|---|
| With calcium chloride; calcium oxide In neat (no solvent) mixt. with flux and binder using stirrer; flux was CaCl2 or CaO powder; binder collodion soln. consisted of 5 mass% nitrocellulose in ethanol and ether; | A 99%
B n/a |
-
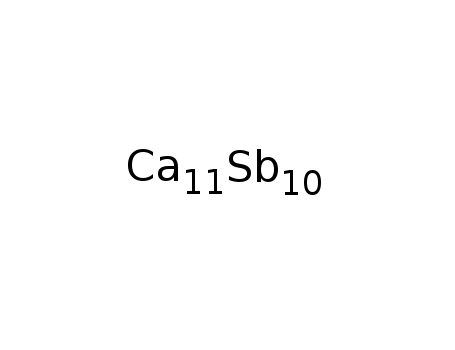
-
Ca11Sb10
Conditions
| Conditions | Yield |
|---|
| Electric Arc; Sr:Sb=1:1; | 99% |
| In melt 5:4-mixt. of Ca and Sb heated in alumina or Ta crucible to 1100°C, kept at this temp. for ca. 1 h, cooled with 50-100°C; | |
| With Sn In melt byproducts: Ca5Sb3; Ca:Sb:Sn were layered in Al2O3 crusible in the ratio 11:10:50; the mixt.was heated under Ar to 1275 K; the react. was held at this temp. for 1 h and cooled at a rate of 277 K/h to 975 K; the single-crystal compd. was sepd. from the molten Sn; identified by powder XRD studies; | |
-
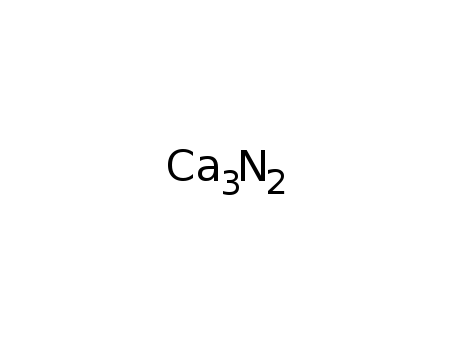
-
calcium nitride
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) (Ar); placing of cleaned Ca rod in alumina crucible inside a molybdenum foil-lined reaction tube; heating under flowing N2 for 48 h at 1000°C; | 98% |
| In neat (no solvent) heating of electrolyt-Ca-chips in a N2-stream at 660°C for 1/2 h; cooling down, grinding; heating at approx. 850°C for 1/2 h in a N2-stream;; soiled with 3.24% unreacted Ca and some CaO;; | 90.9% |
| In neat (no solvent) heating of electrolyt-Ca-chips in a N2-stream at 660°C for 1/2 h; cooling down, grinding; heating at approx. 850°C for 1/2 h in a N2-stream;; soiled with 3.24% unreacted Ca and some CaO;; | 90.9% |
-
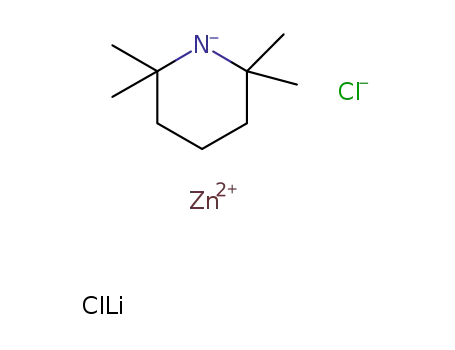
-
zinc chloride-2,2,6,6-tetramethylpiperidin-1-ide lithium chloride complex
-
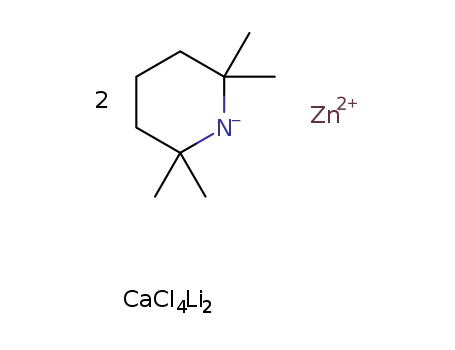
-
2C9H18N(1-)*Zn(2+)*CaCl2*2LiCl
Conditions
| Conditions | Yield |
|---|
| With iodine In tetrahydrofuran at 25℃; for 24h; Schlenk technique; Inert atmosphere; | 98% |
-
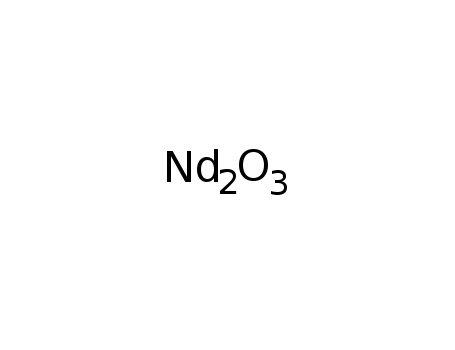
-
neodymium(III) oxide
-
A
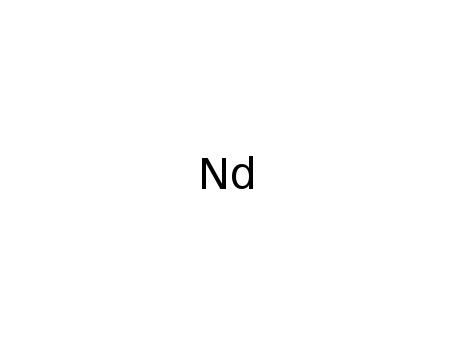
-
neodymium
-
B

-
calcium oxide
Conditions
| Conditions | Yield |
|---|
| In melt metallothermic redn. of Nd2O3 with Ca in CaCl2-NaCl melt at temps. between 983 and 1063 K; Nd recovered from the salt melt by dissoln. in a molten mtal pool of either Nd-Zn or Nd-Fe; vac. distn. of the Nd-Zn alloy; | A 95%
B n/a |
-
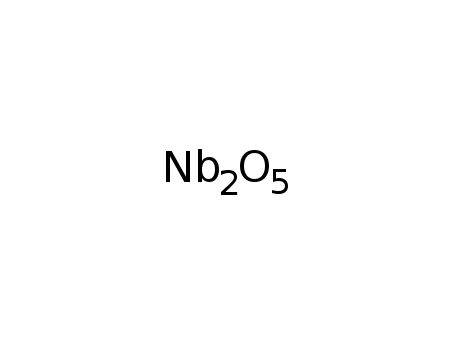
-
niobium(V) oxide
-

-
niobium
Conditions
| Conditions | Yield |
|---|
| extracting with HCl; | 95% |
| extracting with HCl; | 95% |
| best yield with 50 % excess of Ca, with S as react. booster; purity: 99.5 % Nb; | |
-
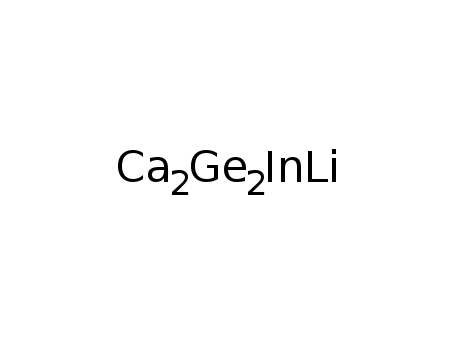
-
Ca2LiInGe2
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) the mixt. of metals in sealed tantalum ampules was heated to 1050°C and held for 10 h, slow cooled over 5 days to about 300°C (Ar atm.); | 95% |
-

-
graphite
-
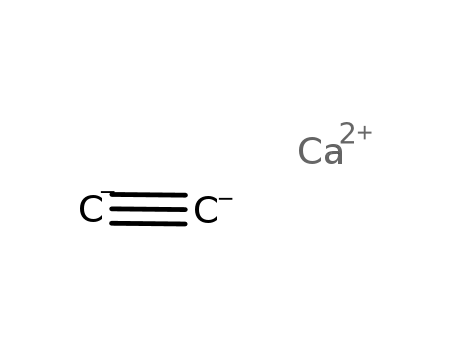
-
calcium carbide
-
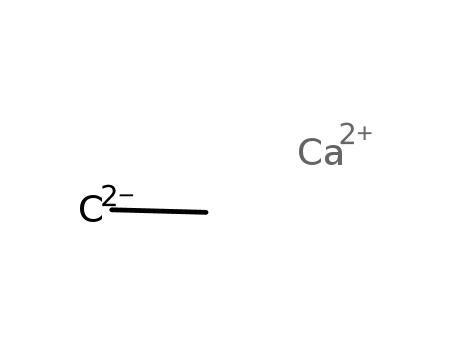
-
calcium carbide
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent, solid phase) all manipulations under dry Ar atm.; Ca and C (1:2.2) heated at 925°C for 16 h, then cooled; speed of haeting and cooling 5°C/min; | A 95%
B n/a |
| In neat (no solvent, solid phase) all manipulations under dry Ar atm.; Ca and C (1:1.8) heated at 925°C for 16 h, then cooled; speed of heating and cooling 5°C/min; | A n/a
B 90% |
-

-
calcium germanide
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent, solid phase) placing of Ca and Ge in welded Ta containers, heating under vac. at 1150°C for 6 h, cooling to 650°C with rate of 10-12°C/h; | 95% |
| In melt stoich. mixt. melted under Ar; XRD; | |
-
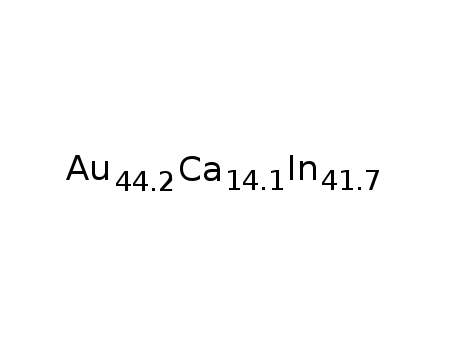
-
Ca14.1Au44.2In41.7
Conditions
| Conditions | Yield |
|---|
| In melt Electric Arc; (Ar); arc melting mixt. of calcium, gold and indium; | 95% |
| In melt (Ar); heating mixt. of calcium, gold and indium at 800°C for 24 h; quenching; | |
-
![bis(tetrahydrofuran)calcium-bis[tris(trimethylsilylmethyl)zincate]](//file1.lookchem.com/cas/reactions/2021/08/20/17636801.png_ms)
-
bis(tetrahydrofuran)calcium-bis[tris(trimethylsilylmethyl)zincate]
Conditions
| Conditions | Yield |
|---|
| In tetrahydrofuran byproducts: Zn; all manipulations under Ar; Ca added to Zn compd. in THF, stirred at room temp. for 24 h; filtered, volatiles evapd. in vac., residue dissolved in toluene, cooledto -20°C for several d, elem. anal.; | 91% |
-
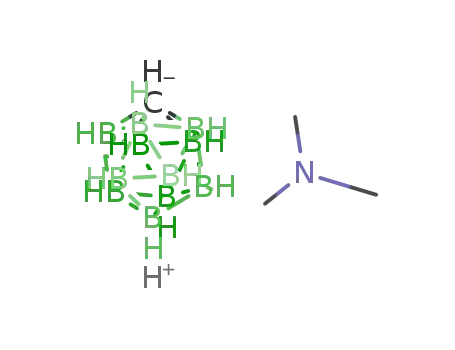
-
trimethylammonium carba-closo-undecahydrododecaborate
-
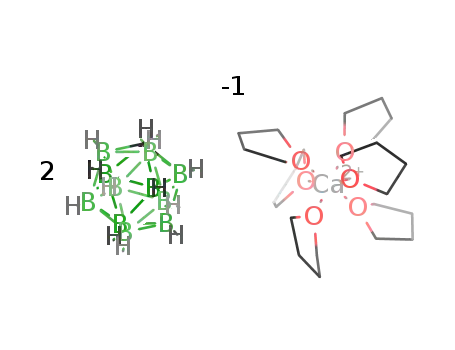
-
2CH12B11(1-)*C24H48CaO6(2+)
-

-
calcium oxide
-

-
Ca3GeO
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) Ar atm.; heating (1100°C, 4 h), cooling (20°C/h); | 90% |
-

-
graphite
-

-
calcium chloride
-
A
-
Ca4 oxy-chloride
-
B
-
Ca3Cl2C3
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent, solid phase) mixture of Ca, CaCl2 and graphite was heated under dry Ar in a sealed tantalum capsule at 900°C for 1 day, annealing at 780°C for 3 days, cooling; | A 5%
B 90% |
-
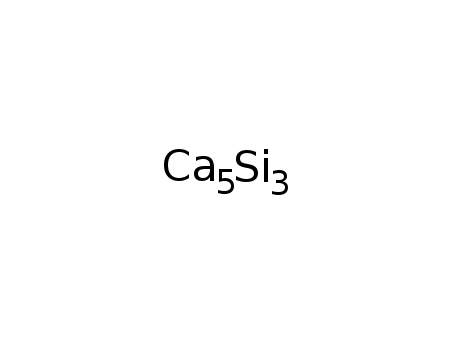
-
calcium silicide
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent, solid phase) placing of Ca and Si in welded Ta containers, heating under vac. at 1300°C for 6 h, cooling to 600°C with rate of 8°C/h; | 90% |
| In melt melt, annealed in vac.; XRD; | |
| In neat (no solvent) sealed, heated at 750 °C for 12 h; | |
-
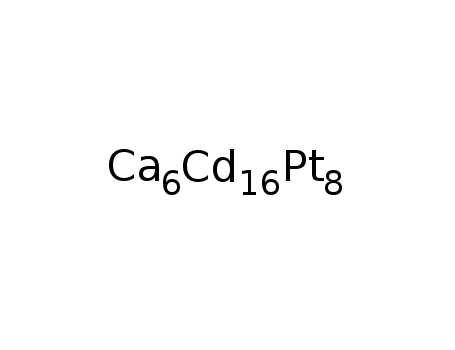
-
Ca6Pt8Cd16
Conditions
| Conditions | Yield |
|---|
Stage #1: calcium; platinum; cadmium In neat (no solvent, solid phase) at 950℃; for 12h; Sealed tube;
Stage #2: In neat (no solvent, solid phase) at 500℃; for 240h; Time; Temperature; Sealed tube; | 90% |
-
![[(IP-)2Al]2(μ2η3-OCO2)](//file1.lookchem.com/cas/reactions/2021/08/20/23731519.png_ms)
-
[(IP-)2Al]2(μ2η3-OCO2)
-
A
![Bu4N[(IP(-))2Al]](//file1.lookchem.com/cas/reactions/2021/08/20/21811489.png_ms)
-
Bu4N[(IP(-))2Al]
-
B

-
calcium carbonate
Conditions
| Conditions | Yield |
|---|
Stage #1: [(IP-)2Al]2(μ2η3-OCO2); calcium In tetrahydrofuran at 60℃; for 1h; Inert atmosphere;
Stage #2: tetra-(n-butyl)ammonium iodide In diethyl ether for 24h; Inert atmosphere; | A 89%
B 90% |
-

-
NbO1.4
-

-
niobium
Conditions
| Conditions | Yield |
|---|
| with 60 % excess of Ca, 1000°C; purity: 98 % Nb; | 89% |
| with 60 % excess of Ca, 1000°C; purity: 98 % Nb; | 89% |
-

-
C9H18N(1-)*Cl(1-)*Zn(2+)*CaCl2
Conditions
| Conditions | Yield |
|---|
| With iodine; diisobutylaluminium hydride In tetrahydrofuran at -5 - 25℃; for 0.5h; Schlenk technique; Inert atmosphere; | 88% |
-
![Ca(2+)*2Al(3+)*8(CH3)2CHO(1-)=Ca[Al(OCH(CH3)2)4]2](//file1.lookchem.com/cas/reactions/2021/08/20/17444133.png_ms)
-
Ca(2+)*2Al(3+)*8(CH3)2CHO(1-)=Ca[Al(OCH(CH3)2)4]2
Conditions
| Conditions | Yield |
|---|
| mercury dichloride In isopropyl alcohol byproducts: H2; absence of moisture; 2 equiv. Al(OiPr)3, refluxing for 120 h; evapn. (reduced pressure), sublimation (reduced pressure); elem. anal.; | 86% |
-
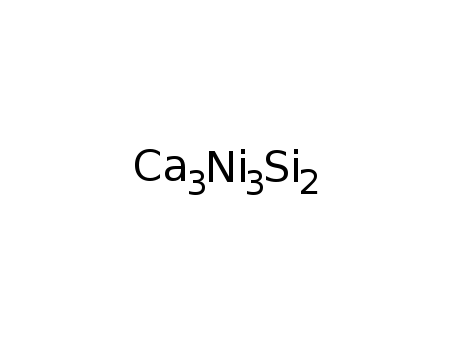
-
Ca3Ni3Si2
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent, solid phase) al manipulations under Ar; mixt. of elements filled into Ta ampouls, heated at 1000°C for 16 h, then at 800°C for 3 d; cooled (5 K/min); | 85% |
-

-
graphite
-
A

-
calcium(II) sulfide
-
-
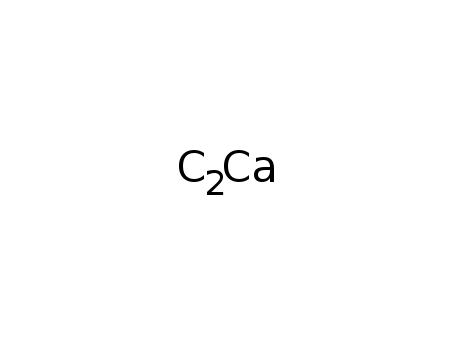
calcium carbide
Conditions
| Conditions | Yield |
|---|
| With S In neat (no solvent, solid phase) High Pressure; (inert atm.), glovebox; addn. of Ca and mixture of C and S (3.77 mass %)into milling vial, milling in 30-60 min increments with 30 min of cooli ng between each increment, in SPEX Certiprep mixer/mills, various times; detected by powder XRD; | A n/a
B 81.1% |
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) melting, hydrogenating (450-460°C, 110 bar); | 80% |
-
-
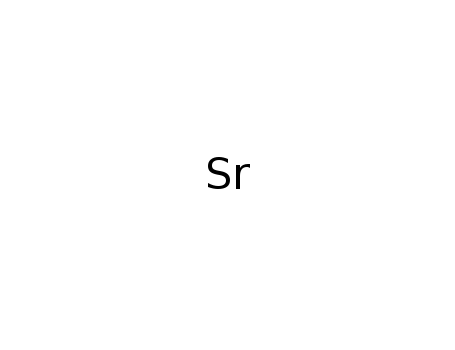
strontium
-
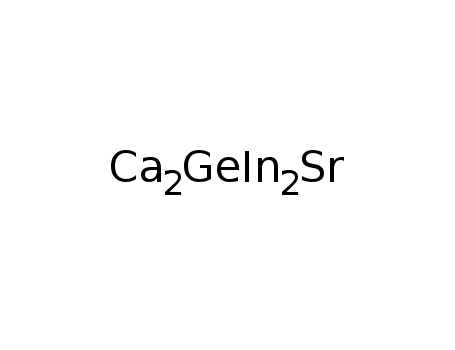
-
SrCa2In2Ge
Conditions
| Conditions | Yield |
|---|
| (Ar), excess In and Ge, heating (350-450°C, 5-8 h, vac.), heating(1050°C, 5 d); ICP analysis; | 80% |
-
-
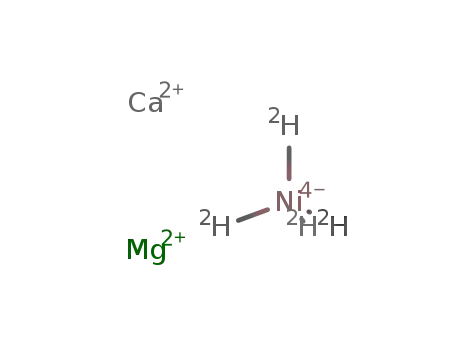
calcium magnesium nickel(0) tetradeuteride
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) melting, hydrogenating (450-460°C, 110 bar); | 80% |
Calcium Chemical Properties
.jpg)
IUPAC Name: Calcium
Empirical Formula: Ca
Molecular Weight: 40.078
EINECS: 231-179-5
Product Categories: Inorganics; Food & Flavor Additives; Calcium Organic Electronics and Photonics; Alkali Metals Metal and Ceramic Science; Electrode Materials; Metals; Reduction; Substrates and Electrode Materials; Synthetic Reagents
Stability: Stable, but reacts with water to release hydrogen and produce calcium hydroxide. Incompatible with strong oxidizing agents, alcohols, moisture.
Melting Point: 850 °C(lit.)
density: 1.54 g/mL at 25 °C(lit.)
storage temp.: water-free area
Boiling point: 1757 K,1484 °C,2703 °F
Heat of fusion: 8.54 kJ/mol
Heat of vaporization: 154.7 kJ/mol
Specific heat capacity of Calcium (CAS NO.7440-70-2): (25 °C) 25.929 J/mol/K
Calcium History
Calcium (CAS NO.7440-70-2) was known as early as the first century when the Ancient Romans prepared lime as calcium oxide. Literature dating back to 975 AD notes that plaster of paris (calcium sulphate), is useful for setting broken bones. It was not isolated until 1808 in England when Sir Humphry Davy electrolyzed a mixture of lime and mercuric oxide. Davy was trying to isolate calcium; when he heard that Swedish chemist J?ns Jakob Berzelius and Pontin prepared calcium amalgam by electrolyzing lime in mercury, he tried it himself. He worked with electrolysis throughout his life and also discovered/isolated sodium, potassium, magnesium, boron and barium. Calcium metal was not available in large scale until the beginning of the 20th century.
Calcium Uses
Calcium (CAS NO.7440-70-2) is used as a reducing agent in the extraction of other metals, such as uranium, zirconium, and thorium,as a deoxidizer, desulfurizer, or decarbonizer for various ferrous and nonferrous alloys and used as an alloying agent used in the production of aluminium, beryllium, copper, lead, and magnesium alloys. It is used in the making of cements and mortars to be used in construction.,in the making of cheese, where calcium ions influence the activity of rennin in bringing about the coagulation of milk.
Calcium Safety Profile
Hazard Codes:  F
F
Risk Statements: 15
R15:Contact with water liberates extremely flammable gases.
Safety Statements: 8-24/25-43
S8:Keep container dry.
S24/25:Avoid contact with skin and eyes.
S43:In case of fire use ... (there follows the type of fire-fighting equipment to be used.)
RIDADR: UN 1401 4.3/PG 2
WGK Germany: 1
HazardClass: 4.3
PackingGroup: II
Calcium Standards and Recommendations
DOT Classification: 4.3; Label: Dangerous When Wet
Calcium Analytical Methods
For occupational chemical analysis use NIOSH: Calcium, 7020.
Calcium Specification
Calcium (CAS NO.7440-70-2), its Synonyms are Elemental calcium ; HSDB 273 ; UNII-SY7Q814VUP . It is silvery, soft metal that turns grayish white on exposure to air. Calcium is a soft gray alkaline earth metal, and is the fifth most abundant element by mass in the Earth's crust. Calcium is also the fifth most abundant dissolved ion in seawater by both molarity and mass, after sodium, chloride, magnesium, and sulfate.
 F
F































































































.jpg)
 F
F