Refine
 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
Synthetic route

-
-
7440-09-7
potassium
Conditions

| Conditions | Yield |
|---|---|
| With pyrographite In neat (no solvent) reduction of a small amount KOH starts at 700°C (in vac.) and proceeds at 900 °C;; | 90% |
| With pyrographite In neat (no solvent) reduction of a small amount KOH starts at 700°C (in vac.) and proceeds at 900 °C;; | 90% |
| With pyrographite In neat (no solvent) KOH is reduced by C at lower temp. than by Fe;; |

-
-
7440-09-7
potassium
Conditions

| Conditions | Yield |
|---|---|
| With zirconium In neat (no solvent) K2WO4 + Zr (1:4), start of react.: 570 °C, calm react. process, yield: about 80% K, no oxide;; | 80% |
| With zirconium In neat (no solvent) pressed powder mixture (K2WO4:Zr = 1:4), heating in vac. to 570°C; mostly explosive reaction, with great excess of Zr smoothy;; further products;; | |
| With Zr In neat (no solvent) pressed powder mixture (K2WO4:Zr = 1:4), heating in vac. to 570°C; mostly explosive reaction, with great excess of Zr smoothy;; further products;; |
Conditions

| Conditions | Yield |
|---|---|
| With zirconium In neat (no solvent) react. of pressed powd. K2CrO4/Zr mixt. (weight ratio 1:2 and 1:4) at 800 °C;; | A 0% B 80% |
-
-
20762-60-1
potassium azide

-
-
7440-09-7
potassium
Conditions

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: N; heating 10-12g KN3 (fine powder) to 355°C (high vacuum, electrically heated tube from instrument glass (Jena)), decompn. starts sometimes 3-4h after obtaining temp., synthesis lasts some d;; spectroscopically pure and gas-free K obtained;; | 80% |
| In neat (no solvent) byproducts: N; thermal decompn. of KN3 (above 350°C, formation of N);; | |
| In neat (no solvent) byproducts: N; thermal decompn. of KN3 begins at 320°C and proceeds continually at 360°;; |

-
-
7440-09-7
potassium
Conditions

| Conditions | Yield |
|---|---|
| With iron In neat (no solvent) byproducts: Fe2O3, FeS; heating react. mixture in a special apparatus for 2 h at 1000 °C, 1mm Hg; using of rough Fe-splints possible; further by-products: SO2 and O2;; | 80% |
| With iron In neat (no solvent) reductn. of K2SO4 by Fe in a quartz pot at 875 up to 1300 °C;; impured with 10 % sulfide;; | 60% |
| With zirconium In neat (no solvent) byproducts: K-oxide, K-sulfide; K2SO4 + Zr (1:4), start of react.: 725 °C, explosion, yield: 20% K, 20% K-oxide, 20% K-sulfide;; | 20% |
Conditions

| Conditions | Yield |
|---|---|
| redn. with Zr powder in vac., 570°C; | 80% |
| redn. with Zr powder in vac., 570°C; | 80% |

-
-
7440-09-7
potassium
Conditions

| Conditions | Yield |
|---|---|
| With zirconium In neat (no solvent) heating of K2MoO4 and Zr powder (1:4) in vac. at 500°C;; | 70% |
| With zirconium In neat (no solvent) K2MoO4 + Zr (1:4), start of react.: about 500 °C, calm react. process, yield: about 70% K, no oxide;; | 70% |
| With Zr In neat (no solvent) heating of K2MoO4 and Zr powder (1:4) in vac. at 500°C;; | 70% |
| With zirconium In neat (no solvent) pressed powder mixture (K2MoO4:Zr = 1:4), heating in vac. to about 500°C; mostly explosive reaction, with great excess of Zr smoothy;; further products;; | |
| With Zr In neat (no solvent) pressed powder mixture (K2MoO4:Zr = 1:4), heating in vac. to about 500°C; mostly explosive reaction, with great excess of Zr smoothy;; further products;; |

-
-
7440-09-7
potassium
Conditions

| Conditions | Yield |
|---|---|
| With iron In neat (no solvent) byproducts: Fe-oxide, Fe-arsenide; heating of react. mixture in a special apparatus at 900 °C, 1mm Hg for 8h;; very pure and As-free K obtained;; | 50% |

-
-
7440-09-7
potassium
Conditions

| Conditions | Yield |
|---|---|
| With zirconium In neat (no solvent) byproducts: K-oxide; K2Cr2O7 + Zr (1:4), start of react.: 370 °C, explosion, yield: 40% K, 30% oxide;; | 40% |
| With zirconium In neat (no solvent) pressed powder mixture (K2Cr2O7:Zr = 1:10), heating in vac. to 380°C; mostly explosive reaction, with great excess of Zr smoothy;; further products;; | |
| With aluminium In neat (no solvent) on ignition of a mixture of K2Cr2O7 and Al; vaporization of K;; |
Conditions

| Conditions | Yield |
|---|---|
| With iron In neat (no solvent) byproducts: Fe2O3, CO, CO2; heating react. mixture in a special apparatus for 2 h at about 1000 °C, 1mm Hg; sucking off CO, CO2 (formation of explosive compounds possible);; | >99 |
| With aluminium In neat (no solvent) byproducts: Al2O3, C; heating of K2CO3 with Al in a stream of H2 to red heat; no potassium carbonyl is formed;; | >99 |
| With pyrographite In neat (no solvent) byproducts: CO; reduction of K2CO3 by C; no formation of K2O as intermediate;; |
...Expand

-
-
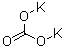
584-08-7
potassium carbonate

-
-
7429-90-5
aluminium

-
A
-
1333-84-2, 1344-28-1
aluminum oxide

-
B
-
7440-09-7
potassium
Conditions

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: C; heating equal amounts of carbonate and Al powder to red heat;; powdery product mixture obtained;; | |
| In neat (no solvent) byproducts: C; heating equal amounts of carbonate and Al powder to red heat;; powdery product mixture obtained;; |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| With pyrographite |

-
-
7440-09-7
potassium
Conditions

| Conditions | Yield |
|---|---|
| With zirconium In neat (no solvent) pressed powder mixture (K2CrO4:Zr = 1:4), heating in vac. to 800°C; mostly explosive reaction, with great excess of Zr smoothy;; further products;; | |
| With zirconium In neat (no solvent) K2CrO4 + Zr (1:4), start of react.: 800 °C, calm react.process, yield: up to 80% K, no oxide; especially appropriate for direct preperation of small amount of K in apparatus (at low temp.);; | <=80 |
| With Zr In neat (no solvent) pressed powder mixture (K2CrO4:Zr = 1:4), heating in vac. to 800°C; mostly explosive reaction, with great excess of Zr smoothy;; further products;; |

-
-
7440-09-7
potassium
Conditions

| Conditions | Yield |
|---|---|
| With magnesium In neat (no solvent) heating a mixt. of 50 g dried KF and 18 g Mg gravel in an iron retort at pressure of 11 mm to 500 - 700 °C;; K distills off at 540 °C into a paraffine filled receiver; yield: 48 g;; | |
| With aluminium In neat (no solvent) at m. p. of KF with Al pieces (use of Al powder is dangerous);; K is distilled off; pure;; | |
| With calcium In neat (no solvent) at red heat;; |
Conditions

| Conditions | Yield |
|---|---|
| In neat (no solvent) on strong heating;; | |
| In neat (no solvent) on strong heating;; |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| With iron In neat (no solvent) byproducts: Fe-halogenide; special apparatus used, 900 - 1000 °C, 1mm Hg; yield: small amount of K;; |
Conditions

| Conditions | Yield |
|---|---|
| Kinetics; Ar-carrier gas, at 3020 K; time-resolved absorption; |
Conditions

| Conditions | Yield |
|---|---|
| Kinetics; in system NaBr-K-Na-KBr; | |
| Kinetics; in system NaBr-K-Na-KBr; |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| In nitrobenzene Electrolysis; | A n/a B 0% |
| In nitrobenzene Electrolysis; | A n/a B 0% |
...Expand


-
-
7440-09-7
potassium
Conditions

| Conditions | Yield |
|---|---|
| With barium In neat (no solvent) evapn. of a mixture of a KCl soln. and a 16 % Ba(N3)2 soln. in vac to dryness; residue is heated in high vac.; decompn. of Ba(N3)2 at 100 °C forming finely dispersed Ba which acts as reducing agent: formation of K from KCl on further heating;; small amounts of pure K are obtained quickly;; | |
| With aluminium In neat (no solvent) addn. of Al to molten KCl;; | |
| With aluminium In neat (no solvent) with Al-powder at high temperatures;; |
Conditions

| Conditions | Yield |
|---|---|
| Electrochem. Process; Equilibrium potential at 800 °C: 3.32 V.; |
Conditions

| Conditions | Yield |
|---|---|
| In neat (no solvent) Incomplete reaction.; | |
| In neat (no solvent) Incomplete reaction.; |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| In further solvent(s) Kinetics; solvent: Sn, Bi or Sb; | |
| Kinetics; at 900 °C; in system NaCl-K-Na-KCl; |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| evapn. of reaction mixture formed from BaN6 and alkali chloride to dryness, then reaction at temp. of distn. of product; alkali metal distilles off; | |
| evapn. of reaction mixture formed from BaN6 and alkali chloride to dryness, then reaction at temp. of distn. of product; alkali metal distilles off; |
Conditions

| Conditions | Yield |
|---|---|
| In further solvent(s) Kinetics; solvent: Sn, Bi or Sb; |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| In neat (no solvent) in Ar flow; react. of atomic H with solid KCl and its vapors; detd. by emission spectroscopy and resonance fluorescence; atomic potassium obtained; |

-
-
7440-09-7
potassium
Conditions

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: H; decompn. of KH on heating to red heat in vacuum;; | |
| In neat (no solvent) byproducts: H; decompn. of KH on heating to red heat in vacuum;; |
Conditions

| Conditions | Yield |
|---|---|
| Kinetics; |
Conditions

| Conditions | Yield |
|---|---|
| In neat (no solvent) | 100% |
| differential thermal anal.; | 100% |
...Expand


-
-
7440-09-7
potassium
Conditions

| Conditions | Yield |
|---|---|
| In benzene a stirred soln. of the Fe-compd. and metallic K was heated to 50-60°C for 4 h; filtn., washed with C6H6, dried, elem. anal.; | 100% |

-
-
7440-09-7
potassium
Conditions

| Conditions | Yield |
|---|---|
| In tetrahydrofuran (N2 or Ar); stirring over potassium mirror, 12 h; filtering, concg. in vac.; | 100% |
Conditions

| Conditions | Yield |
|---|---|
| In neat (no solvent, solid phase) under Ar; mixt. was heated to 523 K for 1 d, then heated at 1023 K for 1d, then cooled to 773 K at 2 K/h, kept at 773 K for 3 d, then cooled to 473 K at 2 K/h in 5 d before switching off the furnace; XRD; | 100% |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| In neat (no solvent, solid phase) under Ar; mixt. was heated to 523 K for 1 d, then heated at 973 K for 1 d, then cooled to 773 K at 2 K/h, kept at 773 K for 3 d, then cooled to 473 K at 2 K/h in 5 d before switching off the furnace; XRD; | 100% |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| In toluene (N2) Hf complex, K and 18-crown-6 in toluene were stirred for 24 h; pentane was added, ppt. was filtered off; elem. anal.; | 100% |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| In benzene byproducts: KCl; exclusion of moisture, slow addn. of a soln. of KAl(OPr(i))4 (K, Al(OPr(i))3 and OHCH(CH3)2 in C6H6, reflux, 2 h) to a suspension of the Y-salt in C6H6, stirring, 60°C, addn. of KOPr(i) (K, HOCH(CH3)2 in C6H6) stirring, molar ratio 1:1:2 Y:KAl:K; recrystn. (n-hexane) yielding 60%; | 99% |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| In diethyl ether; isopropyl alcohol byproducts: KCl, Hg(Oi-Pr)2; K dissolved in i-PrOH, Al(Oi-Pr)3 added, this soln. is added to an ether soln. of the metal chloride, mixt. stirred for ca. 2 h at 0 °C, Hg:Al:K=1:1:2; filtered; elem anal.; | 99% |
| In diethyl ether; isopropyl alcohol byproducts: KCl; K dissolved in i-PrOH, Al(Oi-Pr)3 added, this soln. is added to an ether soln. of the metal chloride, mixt. stirred for ca. 2 h at 0 °C, Hg:Al:K=1:2:2; filtered; elem anal.; | 97% |
...Expand

-
-
156219-63-5, 159861-02-6
[(CH3)Zn((CH3)3CNCHCHNC(CH3)3)]2

-
-
7440-09-7
potassium
Conditions

| Conditions | Yield |
|---|---|
| In tetrahydrofuran N2-atmosphere; stirring (room temp., 16 h); evapn. (vac.), washing (Et2O), drying (vac.); | 99% |
| In tetrahydrofuran (inert atmosphere); stirring (room temp., 16 h); solvent removal (vac.), washing (hexanes), drying (vac.); elem. anal.; | 99% |
-
-
866338-41-2
(tetramethylcyclopentadienyl)2UMe2

-
-
7440-09-7
potassium
Conditions

| Conditions | Yield |
|---|---|
| In toluene (Ar); Schlenk technique; toluene was added to K; mixt. was vigorously stirred under heating; soln. of U complex in toluene was added dropwise; mixt. was cooled to room temp. for 3 d; solvent removed; residue washed (toluene); washings discarded; residue dried; elem. anal.; | 99% |
-
-
191107-88-7
C2B3(OCH3)(Si(CH3)3)2(C6H(CH3)4)2

-
-
7440-09-7
potassium
Conditions

| Conditions | Yield |
|---|---|
| In diethyl ether | 99% |
Conditions

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 130℃; for 3h; Heating; | 99% |
| at 120℃; for 2h; Inert atmosphere; Schlenk technique; Heating; | |
| In neat (no solvent, solid phase) at 20℃; Inert atmosphere; |
Conditions

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -78 - 20℃; Schlenk technique; Inert atmosphere; | 99% |
Conditions

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: KCl; anhydrous atmosphere; refluxing K with 3,5-dimethylphenol, then addn. to Zr-complex (molar ratio Zr:phenolate=1:2), refluxing and stirring (12 h, room temp.); KCl filtered off, concentration, recrystn. (THF, hexane); elem. anal.; | 98% |
...Expand

-
-
139055-84-8
H(Sb(ClCH2CH(O)CH(O)CH2Cl)2)

-
-
7440-09-7
potassium
Conditions

| Conditions | Yield |
|---|---|
| With methanol In methanol refluxing potassium in methanol, addn. of the soln. to a suspn. of the antimony compd., refluxing for 1.5 h; all manipulations under anhydrous atmosphere; concg. under reduced pressure, elem. anal.; | 98% |
Conditions

| Conditions | Yield |
|---|---|
| In benzene byproducts: KCl; exclusion of moisture, slow addn. of a soln. of KAl(OPr(i))4 (prepared from K, Al(OPr(i))3 and isopropanol in benzene, reflux, 2 h) to a suspension of the Y salt in benzene with stirring, 60°C, 5 h, then stirring overnight, ca 30°C; filtration of KCl, removal of volatiles under reduced pressure (30°C/ 1 Torr), recrystn. (n-hexane-CH2Cl2 (2:1)) yielding ca 60%, elem. anal.; | 98% |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| In isopropyl alcohol; benzene byproducts: KCl; treatment of K with Al(Oi-Pr)3 in mixt. isopropanol/benzene, addn. to suspn. of FeCl3 in benzene (molar ratio K/Al(Oi-Pr)3/FeCl3 2:2:1, stirring), refluxing (12 h); sepn. of pptd. KCl (filtration off), removal of solvent (vac.); elem. anal.; | 98% |
...Expand

-
-
28227-42-1, 30834-74-3
1,4-di-tert-butyl-1,4-diazabutadiene

-
-
544-97-8
dimethyl zinc(II)

-
-
7440-09-7
potassium
Conditions

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; pentane N2-atmosphere; stirring (room temp., 16 h); evapn. (vac.), washing (hexanes or Et2O), drying (vac.); elem. anal.; | 98% |
...Expand

-
-
191107-87-6
C2B3(N(Si(CH3)3)2)(Si(CH3)3)2(C6H(CH3)4)2

-
-
7440-09-7
potassium
Conditions

| Conditions | Yield |
|---|---|
| In diethyl ether | 98% |
Conditions

| Conditions | Yield |
|---|---|
| In toluene (N2) Zr complex, K and 18-crown-6 in toluene were stirred for 24 h; pentane was added, ppt. was filtered off; elem. anal.; | 98% |
...Expand

Conditions

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: KCl; anhydrous atmosphere; refluxing K with 3,5-dimethylphenol, then addn. to Ti-complex (molar ratio Ti:phenolate=1:2), refluxing and stirring (12 h, room temp.); KCl filtered off, concentration, recrystn. (THF, hexane); elem. anal.; | 97% |
...Expand

Related products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View







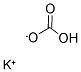





 F,
F, C
C