 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
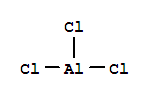
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogen In neat (no solvent) byproducts: HCl; other Radiation; AlCl3 powder placed in quartz tube; tube evacuated and flushed with Ar and then heated under Ar/H2 flow to 90°C; rf discharge ignited for20 min and tube then cooled; XRD; | 100% |
| With potassium In neat (no solvent) heating AlCl3 in a rectangular glass tube and passing the vapor over pieces of K in the horizontal part of the tube;; | |
| With lithium hydride; 1-ethyl-3-methyl-1H-imidazol-3-ium chloride In neat (no solvent) mixed by stirring; deposited at 25-45°C for 15 min - 2 h; |

| Conditions | Yield |
|---|---|
| In benzene at room temp.; | A 92% B n/a |
| In benzene at room temp.; | A 92% B n/a |

-
-
16842-00-5, 855944-65-9
trimethylamine alane

-
-
7429-90-5
aluminium

| Conditions | Yield |
|---|---|
| With hydrogen In 1,2,5-trimethyl-benzene High Pressure; a soln. of Al complex degassed, pressurized with 3 bar of H2, heated to 150°C for 1 h; allowed to settle, the supernatant removed, washed (n-pentane), dried (vac.); obtained as nanoparticles; | 86% |
| With hydrogen In further solvent(s) byproducts: N(CH3)3; High Pressure; pressurized with 3 bar of H2 in mesitylene-d12, heated to 150°C for 1 h; | |
| byproducts: N(CH3)3, H2; film deposition using CVD method (P<1E-6 Torr, gold covered quartz crystal, Teflon, silicon or gallium arsenide as substrates, laser at 5 or 500mW, cooling with liq. N2); SEM; |

| Conditions | Yield |
|---|---|
| In toluene under purified Ar atm.; AlCl3 added to soln. of Ti(η6-MeC6H5)2 in toluene; mixt. stirred for 18 h at room temp.; solid sepd. by filtration; washed (toluene); dried (vac.); identified asAl; soln. evapd. to dryness; solid washed (heptane); dried (vac.); iden tified as Ti-Al complex; | A 80% B 85% |

-
-
79372-14-8
decamethylsamarocene(II) bis(tetrahydrofurane)

-
-
75-24-1
trimethylaluminum

-
A
-
109-99-9
tetrahydrofuran

-
B
-
115756-72-4
(C5Me5)2Sm{(μ-Me)AlMe2(μ-Me)}2Sm(C5Me5)2

-
C
-
7429-90-5
aluminium

| Conditions | Yield |
|---|---|
| In toluene byproducts: methane; all manipulations conducted under nitrogen excluding air and water; after 24 h standing of the reaction mixt. the formed metallic-like ppt. was removed by filtration and washed with hot toluene, filtrates combined, solvent removed by rotary evapn.;; recrystn. (hot toluene), elem. anal.;; | A n/a B 80% C n/a |


| Conditions | Yield |
|---|---|
| In solid SrH2 mechanically treated for 3-4 h, mixed with AlH3 at molar ratio 1:1 in vibrating mechanical load, heated for 3-4 h; DTA-DGV, XRD, IR; | A 80% B n/a C n/a |
| In solid mechanolysis at 165-220°C; DTA-DGV, IR, XRD; |


| Conditions | Yield |
|---|---|
| In neat (no solvent) heating a 1:1 mixture up to 1280°C for 3h; formation of a mixture of the metals and oxides;; | A 64% B n/a |


| Conditions | Yield |
|---|---|
| In toluene under N2, after 3 days heated at 100°C for 15 h; slowly cooled; | A 50% B n/a |


| Conditions | Yield |
|---|---|
| In diethyl ether byproducts: H2; dropwise addn. of AlH3 in ether to soln. of Sm-compd. in diethyl ether, addn. of AlH3*TMEDA, stirred for 24 h; pptn. filtered off, filtrate concd., sepn. after 48 h, washed with pentane, dried in vac.; elem. anal.; | A 35% B n/a |


-
-
60-29-7
diethyl ether

-
-
108-88-3
toluene

-
-
4039-32-1
lithium hexamethyldisilazane


-
B
-
17634-40-1
diethyl ether ; compound with aluminium trichloride

-
C
-
7429-90-5
aluminium

| Conditions | Yield |
|---|---|
| In diethyl ether; toluene byproducts: LiCl*3Et2O; soln. of AlCl in toluene/Et2O (3/1) was added to LiN(SiMe3)2 at -78°C; soln. was warmed to room temp. within 1 d; heated at 60°C for 1 h; filtered; soln. was left at 60°C for 2 mo; pptd.; | A 7% B n/a C n/a |


| Conditions | Yield |
|---|---|
| In neat (no solvent) Kinetics; byproducts: O(C4H9)2, H2; thermal decompn. at 50-100°C; identification of products by IR; | A 5% B n/a |

-
-
60-29-7
diethyl ether

-
-
108-88-3
toluene

-
-
4039-32-1
lithium hexamethyldisilazane


-
B
-
17634-40-1
diethyl ether ; compound with aluminium trichloride

-
C
-
7429-90-5
aluminium

| Conditions | Yield |
|---|---|
| In diethyl ether; toluene byproducts: LiCl*3Et2O; gaseous AlCl were condensed with toluene and diethyl ether at -196°C; soln. of AlCl*Et2O was added to LiN(SiMe3)2 at 25°C; mixt. was left for 1 h; filtered; soln. was heated at 60°C for 1.5 h; pptd.; | A 5% B n/a C n/a |


| Conditions | Yield |
|---|---|
| Stage #1: Na/K alloy; triethylaluminum at 85 - 90℃; for 1.25h; Stage #2: triethylaluminum In toluene at 60 - 80℃; for 4h; Product distribution / selectivity; | |
| Stage #1: Na/K alloy; triethylaluminum at 87 - 95℃; for 3.5h; Stage #2: triethylaluminum In toluene at 20 - 88℃; for 1h; Product distribution / selectivity; | |
| In toluene at 20 - 35℃; for 9.25h; Product distribution / selectivity; |


| Conditions | Yield |
|---|---|
| titanium(III) chloride at 90℃; for 5h; Product distribution / selectivity; Neat (no solvent); Balled milled; | |
| In solid Kinetics; thermal decomposition of ball milled for various times or as-received LiAlH4; powder XRD; | |
| chromium(VI) oxide In diethyl ether; toluene Kinetics; Catalytic decompn. of LiAlH4 in solution; |


| Conditions | Yield |
|---|---|
| titanium(III) chloride at 50 - 150℃; under 760.051 Torr; Product distribution / selectivity; Neat (no solvent); Balled milled; | |
| at 100 - 200℃; under 760.051 Torr; Product distribution / selectivity; Neat (no solvent); Balled milled; | |
| In neat (no solvent) 170°C - 220°C; | |
| With magnesium hydride; titanium(II) hydride In neat (no solvent) at 54 - 171℃; Kinetics; Inert atmosphere; |


| Conditions | Yield |
|---|---|
| titanium(III) chloride at 250 - 350℃; under 760.051 Torr; Product distribution / selectivity; Neat (no solvent); Balled milled; |


| Conditions | Yield |
|---|---|
| titanium(III) chloride at 50 - 200℃; under 760.051 Torr; Product distribution / selectivity; Neat (no solvent); Balled milled; | |
| In neat (no solvent) in stainless steel reactor, at 480-490 K for 3 h, according to: E. C. Ashby, P. Kobertz, Inorg. Chem., 1966, 5, 1615.; XRD; | |
| In neat (no solvent) on heating; | |
| at 210℃; |


| Conditions | Yield |
|---|---|
| titanium(III) chloride at 200 - 250℃; under 760.051 Torr; Product distribution / selectivity; Neat (no solvent); Balled milled; | |
| In neat (no solvent, solid phase) decompd. at heating; |


| Conditions | Yield |
|---|---|
| titanium(III) chloride at 150 - 250℃; under 760.051 Torr; Product distribution / selectivity; Neat (no solvent); Balled milled; | |
| at 200 - 250℃; under 760.051 Torr; Product distribution / selectivity; Neat (no solvent); Balled milled; | |
| In solid 220°C - 275°C; |


| Conditions | Yield |
|---|---|
| titanium(III) chloride at 50 - 250℃; under 760.051 Torr; Product distribution / selectivity; Neat (no solvent); Balled milled; | |
| at 100℃; under 760.051 Torr; Product distribution / selectivity; Neat (no solvent); Balled milled; | |
| at 145 - 305℃; |


| Conditions | Yield |
|---|---|
| hydrogenation at 30 bar H2 at 350°C for 0 to 24 h; detd. by XRD; |


-
-
7429-90-5
aluminium

| Conditions | Yield |
|---|---|
| With iron In neat (no solvent) Al2S3 is decomposed on Fe;; not isolated;; | |
| With iron In neat (no solvent) Al2S3 is molten with iron shavings;; | |
| With H2 or hydrocarbons reduction of Al2S3 by hydrogen or hydrocarbons;; |

| Conditions | Yield |
|---|---|
| byproducts: SO2; | |
| With pyrographite In neat (no solvent) fractionated reduction; first portion: Al alloy with contaminating metalls (Si, Fe, Ti), second portion (using charcoal as reducing reagent):pure Al;; | |
| With pyrographite In neat (no solvent) fractionated reduction; first portion: Al alloy with contaminating metalls (Si, Fe, Ti), second portion (using charcoal as reducing reagent):pure Al;; |

| Conditions | Yield |
|---|---|
| With pyrographite In neat (no solvent) byproducts: graphite; Electric Arc; on heating in an electric arc; formation of Al4C3 containing Al2O3, AlN, Al and graphite;; yield of Al (at best: 30%) increases with decreasing cooling velocity;; | |
| With pyrographite In neat (no solvent) on heating in an electric furnace; formation of Al4C3 containing Al2O3, AlN and Al;; yield of Al (at best: 30%) increases with decreaseing cooling velocity;; |


| Conditions | Yield |
|---|---|
| With caebon In neat (no solvent) byproducts: CO; heating Al2O3 with carbon; formation of solid Al, Al2O3, and Al4C3 as product mixture with residual carbon; reaction mechanism discussed;; | |
| With pyrographite In neat (no solvent) byproducts: CO; Electric Arc; reaction temp. above b.p. of Al at 1 atm; favouring by pressure or by elimination of CO by flushing with H or city gas;; best yield about 49g Al per 106g Al4C3;; | |
| With pyrographite; calcium oxide In neat (no solvent) byproducts: CO; Electric Arc; heating with BaO or Sr-compunds; requirement of energy: 5kWh;; |
-
-
1333-84-2, 1344-28-1
aluminum oxide

-
-
7429-90-5
aluminium

| Conditions | Yield |
|---|---|
| With pyrographite In melt byproducts: CO, CO2; Electrolysis; in molten cryolite; coal-electrodes; decompn. voltage 1.2 V;; | |
| Electrolysis; if Al2O3 contaminated with 0.45% (Fe2O3+SiO2), purity of product 99%, if with 0.15-0.25%, then 99.5%; other impurities: water (<1%), alkali (0.5-2%); | |
| Electrolysis; impurities of Al2O3: water 0.18%, Fe2O3 0.04%, SiO2 0.05%, Na2O 0.18 %; |

| Conditions | Yield |
|---|---|
| In water Al powder activated with HCl; pptn. of Cd; | A n/a B 100% |
| In water Al powder activated with HCl; pptn. of Cd; | A n/a B 100% |
| In water Al wire; incomplete pptn. of Cd; pptn. within some minutes in presence of sodium potassium tartrate; | |
| In water Al wire; incomplete pptn. of Cd; pptn. within some minutes in presence of sodium potassium tartrate; |


| Conditions | Yield |
|---|---|
| In melt in a tantalum tube weld-seald under Ar and protected from air by a silica jacket sealed under vac.; mixt. Li, Al, Si (15:3:6 mol) heated at 1223K, 10 h in vertical furnace and shaken several times;; cooled at rate of 6 K h**-1; elem. anal.; | 100% |


-
-
7429-90-5
aluminium

-
-
115631-72-6
(C4H9)4N(1+)*AlF4(1-) = (C4H9)4NAlF4

| Conditions | Yield |
|---|---|
| In acetonitrile Electrolysis; galvanostatic conditions (300 mA, 10-70 V, 1.7 Ah), aluminum anode, platinum cathode; filtn., evapn. (reduced pressure), recrystn. (Et2O/CHCl3 1:4); elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| In neat (no solvent, solid phase) all manipulations under Ar atm.; stoich. mixt. of compds. sealed in Ta tubes then tubes sealed inside silica ampoules under vac. (ca. 1E-2 mbar), heated at 900°C for 10 d; | 100% |

| Conditions | Yield |
|---|---|
| With Sulfate In further solvent(s) Kinetics; byproducts: NH3, H2; ammine Ni complex reacted with metallic Al in aq. soln. at pH 11.0-12.0; unreacted Al is removed by dissoln. in alkaline soln.; | A n/a B 99.9% |
| With Nitrate In further solvent(s) Kinetics; byproducts: NH3, H2; ammine Ni complex reacted with metallic Al in aq. soln. at pH 11.0-12.0; | A n/a B 0% |

-
-
587-85-9
diphenylmercury(II)

-
-
7429-90-5
aluminium

-
-
7440-66-6
zinc

-
A
-
1078-58-6
diphenylzinc

-
B
-
841-76-9
triphenylaluminium

| Conditions | Yield |
|---|---|
| In neat (no solvent) | A 1% B 99% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) liquid Al reacted with HCl gas in graphite furnace in high vac. at 750°C (by Schnoeckel, H. Z. Naturforsch. 1976, 31b, 1291); | 99% |
| In neat (no solvent) passing HCl gas over liq. Al at 1000°C and 1E-5 mbar; | |
| In neat (no solvent) passing HCl over Al in graphite cell heated to 900°C; |

| Conditions | Yield |
|---|---|
| With hydrogen chloride org. compd. cooling to -80°C, satn. with hydrogen chloride for 3 h, metal turnings addn., mixt. heating under reflux condenser for 5-6 h,crystn. at room temp.; crystals sepn. from mother liquor, washing (isopropyl alcohol), recrystn. from alcohol; elem. anal.; X-ray diffraction; | 99% |

| Conditions | Yield |
|---|---|
| In melt under Ar atm. mixt. Sr and Al was pressed into pellets and arc-melted; | 99% |
| In melt Electric Arc; remelted several times; XRD; | |
| In melt Electric Arc; arc melting of elements with 3 wt. % excess of Sr, remelting four times; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) stoich. amt. of Rb2S5, Al, Ge and S sealed in fused silica tube; heated at 850°C for 3 d; cooled to 400°C over 4 d; detd. by energy-dispersive X-ray analysis; | 99% |


| Conditions | Yield |
|---|---|
| Stage #1: rhodium; aluminium In melt Electric arc; Inert atmosphere; Stage #2: at 999.84℃; for 72h; Sealed tube; Inert atmosphere; Stage #3: at 999.84℃; under 675068 Torr; for 0.333333h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: rhodium; iridium; aluminium In melt Electric arc; Inert atmosphere; Stage #2: at 999.84℃; for 72h; Sealed tube; Inert atmosphere; Stage #3: at 999.84℃; under 675068 Torr; for 0.333333h; Inert atmosphere; | 99% |


| Conditions | Yield |
|---|---|
| Stage #1: rhodium; iridium; aluminium In melt Electric arc; Inert atmosphere; Stage #2: at 999.84℃; for 72h; Sealed tube; Inert atmosphere; Stage #3: at 999.84℃; under 675068 Torr; for 0.333333h; Inert atmosphere; | 99% |


| Conditions | Yield |
|---|---|
| Stage #1: rhodium; iridium; aluminium In melt Electric arc; Inert atmosphere; Stage #2: at 999.84℃; for 72h; Sealed tube; Inert atmosphere; Stage #3: at 999.84℃; under 675068 Torr; for 0.333333h; Inert atmosphere; | 99% |


| Conditions | Yield |
|---|---|
| Stage #1: iridium; aluminium In melt Electric arc; Inert atmosphere; Stage #2: at 999.84℃; for 72h; Sealed tube; Inert atmosphere; Stage #3: at 999.84℃; under 675068 Torr; for 0.333333h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With Na; Al2(C2H5)3Cl3 In not given byproducts: NaCl; NMR spect. anal.; | 98.7% |
| With titanium; hydrogen at 120 - 130℃; under 2250.23 - 75007.5 Torr; for 13h; |

| Conditions | Yield |
|---|---|
| 5h in vac. at 1300-1340 °C; Metal distilles off. Repeated distn. gives 99.5% purity; | 98.5% |
| 4h in vac. at 1010-1030 °C; opening of apparatus under CO2, crushing of product under desiccated toluene to avoid self inflammation; Metal sublimes off. Repeated distn. gives 99.48% purity; | |
| sublimation in vac.; 99.9% Ba; | |
| sublimation in vac.; 99.9% Ba; |

| Conditions | Yield |
|---|---|
| With Mg; Na In not given byproducts: MgBr2, NaBr; NMR spect. anal.; | 98.2% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) Al and Ga metals were heated in sealed quartz tube to 700° and then cooled to room temp.; | 98% |
| In neat (no solvent) | |
| In neat (no solvent) | |
| In neat (no solvent) melting Ga and Al in a porcelain tube in vac.;; |

| Conditions | Yield |
|---|---|
| In hexane thin foil of Al was suspended in degassed n-hexane under argon, 1.5 equiv. I2 was added, mixt. was boiled under reflux for 1-3 h; filtered into a heated receiver; elem. anal.; | 96% |
| In further solvent(s) sheet aluminium in I2/ethyl iodide soln.;; impurities of I2;; | |
| In neat (no solvent) addn. of Al to molten I2, ignition and melting of the metal;; |

| Conditions | Yield |
|---|---|
| With catalyst: HgCl2-ZnCl2-MeI In tetrahydrofuran refluxing (60°C, 5 h); addn. of benzene, filtration, evapn.; elem. anal.; | 96% |

| Conditions | Yield |
|---|---|
| With Al; KClO3; CaO In melt Electric Arc; thermit smelting; ball-milled powdered mixt. HfO2, Ta2O5, CaO, CaF2, KClO3 (10:1.2:4:3:6.5 wt.), 15% excess Al was poured inside MgO-lined steelreactor; ignition with burning Mg of trigger mixt. (KClO3:Al=2:1); also in Ar in copper double wall reactor; remelting by electron beam (2.7 kW, 1 h, vac. 3.9E-4 Pa) or arc melting (under Ar, tungsten electrode) to evap. Al excess; elem. anal.; | 95.5% |
| With Al; KClO3; CaO In melt Electric Arc; thermit smelting; ball-milled powdered mixt. HfO2, Ta2O5, CaO, CaF2, KClO3 (10:1.2:4:3:6.5 wt.), 10% excess Al was poured inside MgO-lined steelreactor; ignition with burning Mg of trigger mixt. (KClO3:Al=2:1); also in Ar in copper double wall reactor; remelting by electron beam (2.7 kW, 1 h, vac. 3.9E-4 Pa) or arc melting (under Ar, tungsten electrode) to evap. Al excess; elem. anal.; | 95% |
| With Al; KClO3; CaO In melt Electric Arc; thermit smelting; ball-milled powdered mixt. HfO2, Ta2O5, CaO, CaF2, KClO3 (10:1.2:4:3:6.5 wt.), 5% excess Al was poured inside MgO-lined steel reactor; ignition with burning Mg of trigger mixt. (KClO3:Al=2:1); also in Ar in copper double wall reactor; remelting by electron beam (2.7 kW, 1 h, vac. 3.9E-4 Pa) or arc melting (under Ar, tungsten electrode) to evap. Al excess; elem. anal.; | 85% |


| Conditions | Yield |
|---|---|
| With mercury dichloride In tetrahydrofuran for 24h; Reflux; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With Sn In neat (no solvent) other Radiation; mixt. milled for 2 h; coldly pressed into rods; combustion reaction carried out in CO2 laser; XRD; | 95% |
| In solid self-propagating high-temperature synthesis; |

-
-
97-93-8
triethylaluminum

-
-
7429-90-5
aluminium

-
A
-
75-24-1
trimethylaluminum

-
B
-
2040-00-8
diethylaluminum iodide

| Conditions | Yield |
|---|---|
| With iodine; methyl iodide In ethanol Sonication; N2; CH3I, I2 and Al introduced in a condenser with C2H5OH at -20°C, ultrasonic acceleration at room temp. for 2 h, Et3Al (ratio MeI/Et3Al = 1.50) dropped into soln. within 10 min, sonication for 30 min; distilled (vac.); | A 86% B 95% |


| Conditions | Yield |
|---|---|
| In melt Electric Arc; ingots by arc melting of Ni(75)-Cr(2.5)-Al(20)-W(2.5) (at%), several remelts, sealed in silica tube under vac. with partial pressure of Ar, 1573K (2 weeks), furnace cooled to 1523K, 4 weeks, 1273K (6 weeks), quenched in iced water; electron microscopy, electron probe microanalysis, x-ray diffraction; | A 95% B 5% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) The reactants in sealed tube were heated in an oil bath to 75°C for 1 week;; sublimed at 50°C and 10E-6 torr;; | 95% |

| Conditions | Yield |
|---|---|
| In toluene byproducts: H2; (inert atmosphere); reflux (12-15 h); decantation, extn., centrifugation, distn., recrystn. (benzene); elem. anal.; | 95% |
| In benzene byproducts: H2; (inert atmosphere); reflux (25-30 h); decantation, extn., centrifugation, distn., recrystn. (benzene); elem. anal.; | 87% |

| Conditions | Yield |
|---|---|
| With mercury dichloride for 9h; Reflux; Inert atmosphere; | 95% |
Related products
Raw Materials
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View















 F,
F, Xi,
Xi, Xn
Xn