 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
Synthetic route

| Conditions | Yield |
|---|---|
| With phosgene In acetone at 20℃; for 0.25h; Reagent/catalyst; | 100% |
| With Fe3+-montmorillonite; propionic acid at 25℃; for 6h; | |
| With propionic acid; K 10-montmorillonite; FeCl3; mixture of, dried at 120 degrees C, grounded at 25℃; for 6h; |

| Conditions | Yield |
|---|---|
| Kinetics; Irradiation (UV/VIS); in glass vessel or uviol vessel, wavelenght higher than 2540Å;; | A 100% B 100% |
| Kinetics; Irradiation (UV/VIS); at room temperature, in quartz vessel; equilibrium; wavelenght lower than 2540Å;; | A 92.3% B 92.3% |
| 995 °C; part of a Mg-S-I water splitting cycle; | A 31% B 31% |

| Conditions | Yield |
|---|---|
| In liquid sulphur dioxide under Ar; recrystn. (SO2), elem. anal.; | A 99% B n/a |


-
B
-
7553-56-2
iodine

| Conditions | Yield |
|---|---|
| In neat (no solvent, solid phase) vac.; equimolar ratio, heating (200°C 20 d, 800°C 30 d); XRD; | A n/a B 98.5% C n/a |


| Conditions | Yield |
|---|---|
| In neat (no solvent, solid phase) byproducts: pyridine; heated at 130°C; washed with acetone-water (5:1), dried in vac.; elem. anal.; | A 94% B 96.5% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride byproducts: H2O, H2S, SO2; in presence of KI; | A 90% B n/a |
| With HCl byproducts: H2O, H2S, SO2; in presence of KI; | A 90% B n/a |

-
-
16893-36-0, 83829-87-2, 84277-63-4
{Pt(2)Br2}

-
B
-
7553-56-2
iodine

| Conditions | Yield |
|---|---|
| In neat (no solvent, solid phase) byproducts: pyridine; heated at 120°C; washed with acetone-water (5:1), dried in vac.; elem. anal.; | A 85% B 72.7% |
-
-
14362-44-8
iodide

-
-
7553-56-2
iodine

| Conditions | Yield |
|---|---|
| With ammonium peroxosulfate; iron(III) In hydrogenchloride byproducts: SO4(2-); Irradiation (UV/VIS); at 22°C with UV irradiation for 15 min; subsequent stirring in daylight for 20 min; mechanism discussed;; | 80.2% |
| With ammonium peroxosulfate; iron(III) In hydrogenchloride byproducts: SO4(2-); at 22°C in daylight for 35 min;; | 73.9% |
| With ammonium peroxosulfate; iron(III) In hydrogenchloride byproducts: SO4(2-); at 22°C in the dark for 15 min; subsequent stirring in daylight for 20 min;; | 69.6% |

| Conditions | Yield |
|---|---|
| With HNO3 or I2O5 or H5IO6 In water High Pressure; mixt. of Bi compd., HIO3, HNO3 (or I2O5 or H5IO6) and H2O was sealed in autoclave; heated to 215°C; held for 5 d; cooled at rate 10°C/h to room temp.; decanted; washed (EtOH, H2O); air-dried; | A 75% B n/a |


| Conditions | Yield |
|---|---|
| With triethylamine In ethanol for 4h; | 74% |
-
-
12029-98-0
iodine pentoxide

-
B
-
7553-56-2
iodine

| Conditions | Yield |
|---|---|
| In water High Pressure; UO2, I2O5, KCl, and water were heated in autoclave at 10°C/min to425°C, after 72 h autoclave was cooled over 24 h to 23°C; washed with MeOH and dried; | A 73% B n/a |


| Conditions | Yield |
|---|---|
| In not given in acidic soln.; | 70% |
| In not given in acidic soln.; | 70% |

| Conditions | Yield |
|---|---|
| Stage #1: N-trifluoroacetic-3-nitroaniline With sodium hydride In tetrahydrofuran at 0℃; for 1h; Stage #2: methyl iodide In tetrahydrofuran at 20℃; | 66% |

| Conditions | Yield |
|---|---|
| In water High Pressure; UO2, I2O5, KCl, and water were heated in autoclave to 180°C, after 72 h autoclave was cooled at 9°C/h to 23°C; washed with MeOH and dried; | A n/a B 60% C n/a |

-
-
615-42-9
1,2-Diiodobenzene

-
-
13517-10-7
boron triiodide

-
A
-
7553-56-2
iodine

-
B
-
24633-37-2
5,10-diiodo-5,10-dihydroboranthrene

| Conditions | Yield |
|---|---|
| stirring mixt. of educts at 180°C for 5 h; I2 removed by sublimation at 60°C/0.1 Torr, sublimation of products; | A n/a B 56% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) at room temp.; | 38% |

-
-
7553-56-2
iodine

| Conditions | Yield |
|---|---|
| in presence of O3 at room temp.; | 2.8% |
| in presence of O3 at room temp.; | 2.8% |
| With manganese(IV) oxide at 370°C; |

| Conditions | Yield |
|---|---|
| potassium polymanganite(IV) In solid byproducts: O2; mixt. of KIO3 and potassium polymanganate heated (400-600°C, 2h, air); X-ray diffraction; | A 1% B n/a |
-
-
203255-55-4
2-(iodomethyl)isoindoline-1,3-dione

-
-
7553-56-2
iodine

| Conditions | Yield |
|---|---|
| Zerstzung bei Raumtemperatur; |
-
-
30894-70-3
2,4-dichloro-6-diiodomethyl-[1,3,5]triazine

-
-
7553-56-2
iodine

| Conditions | Yield |
|---|---|
| at 25℃; |
-
-
64761-27-9
8-iodo-3,7-dihydro-purine-2,6-dione

-
-
7553-56-2
iodine

| Conditions | Yield |
|---|---|
| at 200℃; Abspaltung; |

| Conditions | Yield |
|---|---|
| at 130℃; Zersetzung; |
-
-
128475-96-7
(1R)-3-bromo-1,8,8-trimethyl-3-aza-bicyclo[3.2.1]octane-2,4-dione

-
-
10034-85-2
hydrogen iodide

-
-
7553-56-2
iodine

-
-
10034-85-2
hydrogen iodide

-
-
7553-56-2
iodine


| Conditions | Yield |
|---|---|
| at 150℃; |
-
-
7446-09-5
sulfur dioxide

-
-
7732-18-5
water

-
-
7553-56-2
iodine

-
A
-
7664-93-9
sulfuric acid

-
B
-
10034-85-2
hydrogen iodide

| Conditions | Yield |
|---|---|
| 0 - 25 °C; part of a Mg-S-I water splitting cycle; | A 100% B 100% |


| Conditions | Yield |
|---|---|
| In gas | 100% |
| In solid | 100% |
| In further solvent(s) with I2 dissolved in organic solvents; | 100% |


| Conditions | Yield |
|---|---|
| In melt passing a stream of I2/inert gas into molten Bi with formation of volatile BiI3; description of the aparatus given;; | 100% |
| In melt passing a stream of I2/inert gas into molten Bi with formation of volatile BiI3; description of the aparatus given;; | 100% |
| In melt passing a stream of I2/inert gas into molten Bi with formation of volatile BiI3; description of the aparatus given;; | 100% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) in vac., for 0% excess Tl at 540°C, for 1% excess Tl at 500, 540°C, for 2, 5, 10, 20% excess Tl at 450, 500, 540°C; sublimed, elem. anal., IR, XRD; | 100% |
| In neat (no solvent) in vac., for 20% excess Tl at 400°C; sublimed, elem. anal., IR, XRD; | 99.1% |
| In neat (no solvent) in vac., for 0% excess Tl at 500°C; sublimed, elem. anal., IR, XRD; | 98.9% |

| Conditions | Yield |
|---|---|
| Kinetics; byproducts: H2, LiI; Interaction of initial cryst. materials in molar ratio LiBH4:I2 = 1.50 under mechanical activation with a vibration ball mill (dry N2, atm. pressure, 25°C).; Detn. of gas phase formed by infrared and mass spectroscopy, and gas volumetric anal.; | 100% |
| byproducts: H2, LiI; Interaction of initial cryst. materials in molar ratio LiBH4:I2 = 1.35 under mechanical activation with a vibration ball mill (dry N2, atm. pressure, 25°C).; Detn. of gas phase formed by infrared and mass spectroscopy, and gas volumetric anal.; | 99.5% |
| Kinetics; byproducts: H2, LiI; Interaction of initial cryst. materials in molar ratio LiBH4:I2 = 2.0 under mechanical activation with a vibration ball mill (dry N2, atm. pressure, 25°C).; Detn. of gas phase formed by infrared and mass spectroscopy, and gas volumetric anal.; | 99.8% |
| Kinetics; byproducts: H2, LiI; Interaction of initial cryst. materials in molar ratio LiBH4:I2 = 2.5 under mechanical activation with a vibration ball mill (dry N2, atm. pressure, 25°C).; Detn. of gas phase formed by infrared and mass spectroscopy, and gas volumetric anal.; | 95.4% |
| byproducts: H2, HI; Grinding of initial materials (molar ratio = 1) while being cooled withliq. N2, thorough mixing, transferring of mixt. to a thermographic ampoule and heating in vac. or N2.; Sepn. of gas mixt. by fractional condensation. Detn. of composition after the thermography by gas volumetric anal.; |

| Conditions | Yield |
|---|---|
| byproducts: H2, NaI; Interaction of initial cryst. materials in molar ratio NaBH4:I2 = 1.35 under mechanical activation with a vibration ball mill (dry N2, atm. pressure, 25°C).; Detn. of gas phase formed by infrared and mass spectroscopy, and gas volumetric anal.; | 100% |
| byproducts: H2, NaI; Interaction of initial cryst. materials in molar ratio NaBH4:I2 = 1.5 under mechanical activation with a vibration ball mill (dry N2, atm. pressure, 25°C).; Detn. of gas phase formed by infrared and mass spectroscopy, and gas volumetric anal.; | 97.7% |
| Kinetics; byproducts: H2, NaI; Interaction of initial cryst. materials in molar ratio NaBH4:I2 = 2.5 under mechanical activation with a vibration ball mill (dry N2, atm. pressure, 25°C).; Detn. of gas phase formed by infrared and mass spectroscopy, and gas volumetric anal.; | 94% |
| Kinetics; byproducts: H2, NaI; Interaction of initial cryst. materials in molar ratio NaBH4:I2 = 2.0 under mechanical activation with a vibration ball mill (dry N2, atm. pressure, 25°C).; Detn. of gas phase formed by infrared and mass spectroscopy, and gas volumetric anal.; | 88.7% |
| byproducts: H2, HI; Grinding of initial materials (molar ratio = 1) while being cooled withliq. N2, thorough mixing, transferring of mixt. to a thermographic ampoule and heating in vac. or N2.; Sepn. of gas mixt. by fractional condensation. Detn. of composition after the thermography by gas volumetric anal.; |

-
-
7553-56-2
iodine

-
-
13283-01-7
tungsten(VI) chloride

| Conditions | Yield |
|---|---|
| In neat (no solvent) Ar atmosphere, sealed ampoule, formation of black melt at 300°C, tube furnace (150°C), crystn. (3 months); | 100% |


| Conditions | Yield |
|---|---|
| In dichloromethane first at -30°c, than at room temp. for 10min stirring, reducing of the soln., adding Et2O/pentane; drying at 25°C (vacuo), elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| In tetrachloromethane Kinetics; Irradiation (UV/VIS); Kinetics of the reaction of Co(H2CC6H5)2(bipy)2ClO4 with I2 under irradiation with light at 517 nm is investigated.; PhCh2I and Co(H2CC6H5)I(bipy)2ClO4 are the main products.; | A n/a B 100% |


| Conditions | Yield |
|---|---|
| In tetrachloromethane Irradiation (UV/VIS); Irradiation at 509 nm in CCl4; Estimation of the quantum yield of the photochemical reaction. Yield of the products estimated by g.l.c.; | A 0.02% B <1 C 100% |


| Conditions | Yield |
|---|---|
| In tetrachloromethane Kinetics; byproducts: C2H6, CH4; Irradiation (UV/VIS); Kinetics of the reaction of Co(CH3)2(H3CCNOHC(CH3)NC3H6NC(CH3)CNOCH3) with I2 under irradiation with light at 517 nm is investigated.; MeI and Co(CH3)I(H3CCNOHC(CH3)NC3H6NC(CH3)CNOCH3) are the main products, C2H6 and CH4 are byproducts.; | A n/a B 100% |


| Conditions | Yield |
|---|---|
| In tetrachloromethane Kinetics; Irradiation (UV/VIS); Kinetics of the reaction of Co(CH3)(H3CCNOHONCCH3)2(C5H5N) with I2 under irradiation with light at 517 nm is investigated.; MeI and CoI(H3CCNOHONCCH3)2(C5H5N) are the products.; | A n/a B 100% |


| Conditions | Yield |
|---|---|
| In tetrachloromethane Kinetics; Irradiation (UV/VIS); Kinetics of the reaction of Co(C2H5)(H3CCNOHONCCH3)2(C5H5N) with I2 under irradiation with light at 517 nm is investigated.; MeI and CoI(H3CCNOHONCCH3)2(C5H5N) are the products.; | A n/a B 100% |


-
-
7553-56-2
iodine

| Conditions | Yield |
|---|---|
| With sodium thiosulfate In acetone addn. of iodine to a soln. of Co-compd., stirring at room temp. for 1 h, change of colour from brown-red to red on addn. of aq. Na2S2O3; extg. the aq. layer with CH2Cl2, drying over Na2SO4, filtration, evapn., chromy. (Al2O3, ethyl acetate), elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| In not given react. (N2, CO or Ar); elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| In dichloromethane elem. anal.; | 100% |
-
-
7782-49-2
selenium

-
-
7784-36-3
arsenic pentafluoride

-
-
13637-84-8
fluorosulfonylchloride

-
-
7446-09-5
sulfur dioxide

-
-
7553-56-2
iodine

| Conditions | Yield |
|---|---|
| byproducts: AsF3; SO2 and AsF5 were condensed into Se and I2; after 16 h no insoluble material was observed; condensed SO2ClF into the soln.; react. time 3 h; slowly (ca.20 h) condension; cooling; crystn.; washing; removed volatile materials by evacuation; subjected to vac. for <0.2 h; elem. anal.; | 100% |
| byproducts: AsF3; SO2 and AsF5 were condensed onto Se and I2; after 16 h no insoluble material was observed; condensation of SO2ClF onto the soln.; react. time 7 h; slowly (ca.20 h) condensation of solvent; cooling; crystn.; washing; removal of volatile material by evacuation; elem. anal.; | 104 % |


| Conditions | Yield |
|---|---|
| AsF5 was condensed onto a mixture of Se and I2 in liq. SO2; react. time 4 h; slowly (ca.20 h) condensation of solvent; crystn.; after 16 h removal of volatile material by evacuation; elem. anal.; X-ray diffraction; | 100% |
| AsF5 was condensed onto a mixture of Se and I2 in liq. SO2; react. time 6 h; slowly (ca.20 h) condensation; crystn.; after 16 h removal of volatile materials by evacuation; elem. anal.; X-ray diffraction; | 106 % |

-
-
38573-93-2, 34208-81-6
anti-2-norbornene-7-yl-trimethyl tin

-
-
7553-56-2
iodine

-
-
811-73-4
trimethylstannyl iodide

| Conditions | Yield |
|---|---|
| In methanol | 100% |
| In methanol | 100% |
| In dimethyl sulfoxide | 88% |
-
-
7553-56-2
iodine

-
-
19464-44-9
methyl 3-(tri-n-butylstannyl)propionate

| Conditions | Yield |
|---|---|
| In tetrachloromethane byproducts: CH3(CH2)3I; | 100% |
-
-
136835-13-7
benzimidazolin-2-ylidene(chloro)gold

-
-
7553-56-2
iodine

| Conditions | Yield |
|---|---|
| In dichloromethane protective gas: argon; stirring mixture (20 h/room temp.);; pptn.; filtration; washing (CH2Cl2); drying; elem. anal.;; | 100% |

| Conditions | Yield |
|---|---|
| In not given | 100% |

-
-
7553-56-2
iodine

| Conditions | Yield |
|---|---|
| In nitromethane-d3 not isolated, monitored by NMR; | 100% |
-
-
7553-56-2
iodine

-
-
201533-93-9
bis[2,4,6-tris[bis(trimethylsilyl)methyl]phenyl]distilbene

-
-
201533-95-1
[((Si(CH3)3)2CH)3C6H2]SbI2

| Conditions | Yield |
|---|---|
| In tetrachloromethane room temp.; | 100% |

-
-
7553-56-2
iodine

| Conditions | Yield |
|---|---|
| In further solvent(s) excess of I2, stirring in chloronaphthalene (60-70°C, 1 h); cooling, collection (filtration), washing (EtOH), drying (vac.); elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| In pentane Ar-atmosphere; stirring (40°C, 3 h); detd. by NMR spectroscopy; | 100% |
| In pentane all manipulations with exclusion of air and moisture; soln. of compds. heated at 40°C for 3 h; controlled by NMR; volatiles evapd. by oil pump; elem. anal.; |

| Conditions | Yield |
|---|---|
| In acetonitrile soln. of iodine addn. to soln. of Tl-compd., mixing, standing (dark, 220h, room temperature), evacuation (under Ar, room temperature, then wate r bath, distillation of I-compd.), product remaining in solid residue; | A 99% B 100% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) Ar atm.; molar ratio Te:I2:AlI3 1:2:1, heating (150-200°C, several hours); | 100% |

Related products
Raw Materials
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View





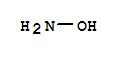




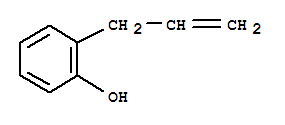





 Xn,
Xn,  N
N