-
Name
Chlorotrifluoromethane
- EINECS 200-894-4
- CAS No. 75-72-9
- Article Data215
- CAS DataBase
- Density 1.434 g/cm3
- Solubility Water: 0.009 % at 25 °C
- Melting Point -181 °C (91.2 K)
- Formula CClF3
- Boiling Point -81.5 °C (191.7 K)
- Molecular Weight 104.459
- Flash Point
- Transport Information UN 1022
- Appearance Colorless gas with sweet odor
- Safety 23-36/37/39
- Risk Codes 20
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Arcton 3;CFC 13;Chlorotrifluoromethane;F 13;FC 13;FKW 13;Freon13;Freon R 13;Frigen 13;Genetron 13;Khladon 13;Monochlorotrifluoromethane;R 13;Refrigerant 13;Refrigerant R 13;Trifluorochloromethane;Trifluoromethyl chloride;Trifluoromonochlorocarbon;
- PSA 0.00000
- LogP 1.74500
Chlorotrifluoromethane Consensus Reports
Reported in EPA TSCA Inventory.
Chlorotrifluoromethane Standards and Recommendations
DFG MAK: 1000 ppm (4300 mg/m3)
DOT Classification: 2.2; Label: Nonflammable Gas
Chlorotrifluoromethane Specification
The Chlorotrifluoromethane with CAS registry number of 75-72-9 is also known as Trifluoromethyl chloride. The IUPAC name is Chloro(trifluoro)methane. It belongs to product categories of CFC; Refrigerants; Organics. Its EINECS registry number is 200-894-4. In addition, the formula is CClF3 and the molecular weight is 104.46. This chemical is a colorless gas with sweet odor and should be stored in ventilated, cool and dry place.
Physical properties about Chlorotrifluoromethane are: (1)ACD/LogP: 1.75; (2)ACD/LogD (pH 5.5): 1.75; (3)ACD/LogD (pH 7.4): 1.75; (4)ACD/BCF (pH 5.5): 12.53; (5)ACD/BCF (pH 7.4): 12.53; (6)ACD/KOC (pH 5.5): 212.53; (7)ACD/KOC (pH 7.4): 212.53; (8)Index of Refraction: 1.261; (9)Molar Refractivity: 11.98 cm3; (10)Molar Volume: 72.8 cm3; (11)Polarizability: 4.75×10-24cm3; (12)Surface Tension: 11.4 dyne/cm; (13)Density: 1.434 g/cm3; (14)Enthalpy of Vaporization: 17.67 kJ/mol; (15)Vapour Pressure: 21000 mmHg at 25 °C.
Preparation of Chlorotrifluoromethane: it is prepared by reaction of dichlorodifluoromethane. Aluminum chloride is used as catalyst in the reaction. What's more, the reaction needs steps such as activation, disproportionation, separation and purification.
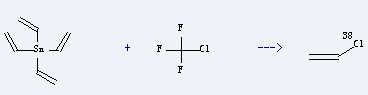
Uses of Chlorotrifluoromethane: it is used as refrigerant and blowing agent. It is used to produce C2H3(38)Cl by reaction with tetravinylstannane. The reaction occurs with irradiation at the temperature of 21.9 °C. The yield is about 95.8%.
When you are using this chemical, please be cautious about it. As a chemical, it is harmful by inhalation. During using it, wear suitable protective clothing, gloves and eye/face protection. What's more, do not breathe gas/fumes/vapour/spray.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C(F)(F)(F)Cl
2. InChI: InChI=1S/CClF3/c2-1(3,4)5
3. InChIKey: AFYPFACVUDMOHA-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View