-
Name
Copper glycinate
- EINECS 236-783-2
- CAS No. 13479-54-4
- Article Data34
- CAS DataBase
- Density 4.39
- Solubility water soluble
- Melting Point 1170 °C
- Formula C4H8CuN2O4
- Boiling Point 240.9 °C at 760 mmHg
- Molecular Weight 211.665
- Flash Point 99.5 °C
- Transport Information
- Appearance
- Safety
- Risk Codes R31; R36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, T
T
- Synonyms Copper,bis(glycinato)- (6CI,8CI);Copper, bis(glycinato-N,O)-;B-Traxim 2C Cu;Bis(aminoacetato)copper;Bis(glycinato)copper;Bis(glycinato)copper(II);CopperChelazome;Copper aminoacetate;Copper bis(glycinate);Copper diglycinate;Copper glycinate;Copper(II) glycinate;Copper(glycine)2;Cupric aminoacetate;Cupric glycinate;Glycine, copper(2+) salt (2:1);NSC 162736;
- PSA 104.64000
- LogP -0.69670
Copper glycinate Specification
This chemical is called Cupric glycinate, and its systematic name is Bis(glycinato)copper. With the molecular formula of C4H8CuN2O4, its molecular weight is 211.66. The CAS registry number of this chemical is 13479-54-4. Additionally, the monohydrate is long deep-blue needles and thedihydrate is light blue powdery crystals.
The properties of the Copper glycinate are: (1)ACD/LogP: -1.03; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -3.53; (4)ACD/LogD (pH 7.4): -3.53; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 1; (8)ACD/KOC (pH 7.4): 1; (9)#H bond acceptors: 3; (10)#H bond donors: 3; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 40.54 Å2; (13)Flash Point: 99.5 °C; (14)Enthalpy of Vaporization: 52.63 kJ/mol; (15)Boiling Point: 240.9 °C at 760 mmHg; (16)Vapour Pressure: 0.0123 mmHg at 25°C.
Production method of this chemical: The Cupric glycinate could be obtained by the reactants of glycine and copper, with the exsistence of magnesium sulfate and copper carbonate.
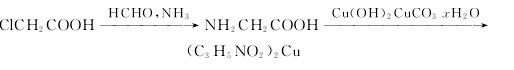
When you are using this chemical, please be cautious about it as the following: Inhalation of dust may cause nasal congestion, conjunctivitis and edema of eyelids. It's irratating. It will cause vomiting by local irritant and astringent action of ionic copper on stomach and bowel.
You can still convert the following datas into molecular structure:
(1)SMILES: [Cu+2].O=C([O-])CN.[O-]C(=O)CN
(2)InChI: InChI=1/2C2H5NO2.Cu/c2*3-1-2(4)5;/h2*1,3H2,(H,4,5);/q;;+2/p-2
(3)InChIKey: VVYPIVJZLVJPGU-NUQVWONBAZ
Related Products
- Copper
- Copper (I) thiocyanate
- Copper (II) fluoroacetate
- Copper acetylide
- COPPER ALLOY, Cu 99.60-100, Cd 0.10-0.30
- COPPER ALLOY, Cu 99.75-100, Cd 0.05-0.15
- Copper alloy, Cu,Be
- COPPER ALLOY, Cu,Cd
- Copper arsenate hydrate
- Copper bis(2-ethylhexanoate)
- 13479-88-4
- 134807-28-6
- 134807-64-0
- 13480-95-0
- 13481-00-0
- 13481-09-9
- 13481-25-9
- 134-81-6
- 13481-70-4
- 13481-87-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View