-
Name
Costunolide
- EINECS
- CAS No. 553-21-9
- Article Data4
- CAS DataBase
- Density 1.03 g/cm3
- Solubility Chloroform (Slightly), Ethyl Acetate (Slightly)
- Melting Point 106-109oC
- Formula C15H20O2
- Boiling Point 385.4 °C at 760 mmHg
- Molecular Weight 232.323
- Flash Point 162 °C
- Transport Information
- Appearance
- Safety 26-24/25
- Risk Codes 36/37/38
-
Molecular Structure
- Hazard Symbols Xi
- Synonyms (1S,4E,8E,10S)-4,8-dimethyl-13-methylidene-11-oxabicyclo[8.3.0]trideca-4,8-dien-12-one;Cyclodeca[b]furan-2(3H)-one,3a,4,5,8,9,11ahexahydro- 6,10-dimethyl-3-methylene-,(3aS,6E,10E,11aR)-;Costunolid;(1S,10S)-4,8-dimethyl-13-methylidene-11-oxabicyclo[8.3.0]trideca-4,8-dien-12-one;Costus lactone;(E,E)-6-alpha-Hydroxygermacra-1(10),4,11(13)-trien-12-oic acid gamma-lactone;
- PSA 26.30000
- LogP 3.55080
Synthetic route

-
-
553-21-9
costunolide

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 20℃; for 12h; Elimination; | 100% |

-
-
553-21-9
costunolide

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 20℃; for 12h; Elimination; | 100% |

-
-
553-21-9
costunolide

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 20℃; for 12h; Elimination; | 100% |
-
-
97317-60-7, 104486-16-0
1S,10R-3,7-dimethyl-10-<2-(1-hydroxy-2-propenyl)>-2E,6E-cyclodecadien-1-ol

-
-
553-21-9
costunolide

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide In dichloromethane at 20℃; for 48h; Inert atmosphere; | 82% |
| With manganese(IV) oxide In dichloromethane at 20℃; for 12h; | 78% |

-
-
553-21-9
costunolide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: carbon tetrabromide; 2,6-dimethylpyridine; triphenylphosphine / dichloromethane / 0.5 h / 0 °C / Inert atmosphere 2: potassium hexamethylsilazane / tetrahydrofuran / 0.25 h / 0 °C / Inert atmosphere 3: magnesium / methanol; tetrahydrofuran / 20 °C / Inert atmosphere 4: pyridinium p-toluenesulfonate / methanol; ethylene glycol / 0.33 h / 20 °C / Inert atmosphere 5: manganese(IV) oxide / dichloromethane / 48 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 5 steps 1: triphenylphosphine; iodine; 1H-imidazole / 0 °C / Inert atmosphere 2: sodium hexamethyldisilazane / tetrahydrofuran / 0.5 h / 0 °C / Inert atmosphere 3: magnesium / methanol; tetrahydrofuran / 20 °C / Inert atmosphere 4: pyridinium p-toluenesulfonate / methanol; ethylene glycol / 0.33 h / 20 °C / Inert atmosphere 5: manganese(IV) oxide / dichloromethane / 48 h / 20 °C / Inert atmosphere View Scheme |

-
-
553-21-9
costunolide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: potassium hexamethylsilazane / tetrahydrofuran / 0.25 h / 0 °C / Inert atmosphere 2: magnesium / methanol; tetrahydrofuran / 20 °C / Inert atmosphere 3: pyridinium p-toluenesulfonate / methanol; ethylene glycol / 0.33 h / 20 °C / Inert atmosphere 4: manganese(IV) oxide / dichloromethane / 48 h / 20 °C / Inert atmosphere View Scheme |

-
-
553-21-9
costunolide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: magnesium / methanol; tetrahydrofuran / 20 °C / Inert atmosphere 2: pyridinium p-toluenesulfonate / methanol; ethylene glycol / 0.33 h / 20 °C / Inert atmosphere 3: manganese(IV) oxide / dichloromethane / 48 h / 20 °C / Inert atmosphere View Scheme |

-
-
553-21-9
costunolide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: pyridinium p-toluenesulfonate / methanol; ethylene glycol / 0.33 h / 20 °C / Inert atmosphere 2: manganese(IV) oxide / dichloromethane / 48 h / 20 °C / Inert atmosphere View Scheme |

-
-
553-21-9
costunolide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 13 steps 1.1: oxalyl dichloride; N,N-dimethyl-formamide / toluene / 0 - 20 °C / Inert atmosphere 1.2: Inert atmosphere 2.1: sodium hydride / toluene; mineral oil / 3 h / 0 - 20 °C / Inert atmosphere 2.2: 2 h / 0 - 20 °C / Inert atmosphere 3.1: 1,1,1,3,3,3-hexamethyl-disilazane; n-butyllithium / tetrahydrofuran; hexane / 2 h / -78 - -40 °C / Inert atmosphere 3.2: 0.08 h / -78 °C / Inert atmosphere 4.1: hydrogenchloride / dichloromethane; ethanol; water / 0.5 h / 0 °C / Inert atmosphere 4.2: Inert atmosphere 5.1: pyridinium p-toluenesulfonate / N,N-dimethyl-formamide / 4 h / 0 - 20 °C / Inert atmosphere 5.2: Inert atmosphere 6.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 0 °C / Inert atmosphere 6.2: 20 °C / Inert atmosphere 7.1: pyridine; hexaammonium heptamolybdate tetrahydrate; dihydrogen peroxide / tert-butyl alcohol / 4 h / 0 - 20 °C / Inert atmosphere 8.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 12 h / 20 °C / Inert atmosphere 9.1: carbon tetrabromide; 2,6-dimethylpyridine; triphenylphosphine / dichloromethane / 0.5 h / 0 °C / Inert atmosphere 10.1: potassium hexamethylsilazane / tetrahydrofuran / 0.25 h / 0 °C / Inert atmosphere 11.1: magnesium / methanol; tetrahydrofuran / 20 °C / Inert atmosphere 12.1: pyridinium p-toluenesulfonate / methanol; ethylene glycol / 0.33 h / 20 °C / Inert atmosphere 13.1: manganese(IV) oxide / dichloromethane / 48 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 13 steps 1.1: oxalyl dichloride; N,N-dimethyl-formamide / toluene / 0 - 20 °C / Inert atmosphere 1.2: Inert atmosphere 2.1: sodium hydride / toluene; mineral oil / 3 h / 0 - 20 °C / Inert atmosphere 2.2: 2 h / 0 - 20 °C / Inert atmosphere 3.1: 1,1,1,3,3,3-hexamethyl-disilazane; n-butyllithium / tetrahydrofuran; hexane / 2 h / -78 - -40 °C / Inert atmosphere 3.2: 0.08 h / -78 °C / Inert atmosphere 4.1: hydrogenchloride / dichloromethane; ethanol; water / 0.5 h / 0 °C / Inert atmosphere 4.2: Inert atmosphere 5.1: pyridinium p-toluenesulfonate / N,N-dimethyl-formamide / 4 h / 0 - 20 °C / Inert atmosphere 5.2: Inert atmosphere 6.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 0 °C / Inert atmosphere 6.2: 20 °C / Inert atmosphere 7.1: pyridine; hexaammonium heptamolybdate tetrahydrate; dihydrogen peroxide / tert-butyl alcohol / 4 h / 0 - 20 °C / Inert atmosphere 8.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 12 h / 20 °C / Inert atmosphere 9.1: triphenylphosphine; iodine; 1H-imidazole / 0 °C / Inert atmosphere 10.1: sodium hexamethyldisilazane / tetrahydrofuran / 0.5 h / 0 °C / Inert atmosphere 11.1: magnesium / methanol; tetrahydrofuran / 20 °C / Inert atmosphere 12.1: pyridinium p-toluenesulfonate / methanol; ethylene glycol / 0.33 h / 20 °C / Inert atmosphere 13.1: manganese(IV) oxide / dichloromethane / 48 h / 20 °C / Inert atmosphere View Scheme |

-
-
553-21-9
costunolide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1.1: 1,1,1,3,3,3-hexamethyl-disilazane; n-butyllithium / tetrahydrofuran; hexane / 2 h / -78 - -40 °C / Inert atmosphere 1.2: 0.08 h / -78 °C / Inert atmosphere 2.1: hydrogenchloride / dichloromethane; ethanol; water / 0.5 h / 0 °C / Inert atmosphere 2.2: Inert atmosphere 3.1: pyridinium p-toluenesulfonate / N,N-dimethyl-formamide / 4 h / 0 - 20 °C / Inert atmosphere 3.2: Inert atmosphere 4.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 0 °C / Inert atmosphere 4.2: 20 °C / Inert atmosphere 5.1: pyridine; hexaammonium heptamolybdate tetrahydrate; dihydrogen peroxide / tert-butyl alcohol / 4 h / 0 - 20 °C / Inert atmosphere 6.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 12 h / 20 °C / Inert atmosphere 7.1: carbon tetrabromide; 2,6-dimethylpyridine; triphenylphosphine / dichloromethane / 0.5 h / 0 °C / Inert atmosphere 8.1: potassium hexamethylsilazane / tetrahydrofuran / 0.25 h / 0 °C / Inert atmosphere 9.1: magnesium / methanol; tetrahydrofuran / 20 °C / Inert atmosphere 10.1: pyridinium p-toluenesulfonate / methanol; ethylene glycol / 0.33 h / 20 °C / Inert atmosphere 11.1: manganese(IV) oxide / dichloromethane / 48 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 11 steps 1.1: 1,1,1,3,3,3-hexamethyl-disilazane; n-butyllithium / tetrahydrofuran; hexane / 2 h / -78 - -40 °C / Inert atmosphere 1.2: 0.08 h / -78 °C / Inert atmosphere 2.1: hydrogenchloride / dichloromethane; ethanol; water / 0.5 h / 0 °C / Inert atmosphere 2.2: Inert atmosphere 3.1: pyridinium p-toluenesulfonate / N,N-dimethyl-formamide / 4 h / 0 - 20 °C / Inert atmosphere 3.2: Inert atmosphere 4.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 0 °C / Inert atmosphere 4.2: 20 °C / Inert atmosphere 5.1: pyridine; hexaammonium heptamolybdate tetrahydrate; dihydrogen peroxide / tert-butyl alcohol / 4 h / 0 - 20 °C / Inert atmosphere 6.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 12 h / 20 °C / Inert atmosphere 7.1: triphenylphosphine; iodine; 1H-imidazole / 0 °C / Inert atmosphere 8.1: sodium hexamethyldisilazane / tetrahydrofuran / 0.5 h / 0 °C / Inert atmosphere 9.1: magnesium / methanol; tetrahydrofuran / 20 °C / Inert atmosphere 10.1: pyridinium p-toluenesulfonate / methanol; ethylene glycol / 0.33 h / 20 °C / Inert atmosphere 11.1: manganese(IV) oxide / dichloromethane / 48 h / 20 °C / Inert atmosphere View Scheme |

-
-
553-21-9
costunolide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1.1: sodium hydride / toluene; mineral oil / 3 h / 0 - 20 °C / Inert atmosphere 1.2: 2 h / 0 - 20 °C / Inert atmosphere 2.1: 1,1,1,3,3,3-hexamethyl-disilazane; n-butyllithium / tetrahydrofuran; hexane / 2 h / -78 - -40 °C / Inert atmosphere 2.2: 0.08 h / -78 °C / Inert atmosphere 3.1: hydrogenchloride / dichloromethane; ethanol; water / 0.5 h / 0 °C / Inert atmosphere 3.2: Inert atmosphere 4.1: pyridinium p-toluenesulfonate / N,N-dimethyl-formamide / 4 h / 0 - 20 °C / Inert atmosphere 4.2: Inert atmosphere 5.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 0 °C / Inert atmosphere 5.2: 20 °C / Inert atmosphere 6.1: pyridine; hexaammonium heptamolybdate tetrahydrate; dihydrogen peroxide / tert-butyl alcohol / 4 h / 0 - 20 °C / Inert atmosphere 7.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 12 h / 20 °C / Inert atmosphere 8.1: carbon tetrabromide; 2,6-dimethylpyridine; triphenylphosphine / dichloromethane / 0.5 h / 0 °C / Inert atmosphere 9.1: potassium hexamethylsilazane / tetrahydrofuran / 0.25 h / 0 °C / Inert atmosphere 10.1: magnesium / methanol; tetrahydrofuran / 20 °C / Inert atmosphere 11.1: pyridinium p-toluenesulfonate / methanol; ethylene glycol / 0.33 h / 20 °C / Inert atmosphere 12.1: manganese(IV) oxide / dichloromethane / 48 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 12 steps 1.1: sodium hydride / toluene; mineral oil / 3 h / 0 - 20 °C / Inert atmosphere 1.2: 2 h / 0 - 20 °C / Inert atmosphere 2.1: 1,1,1,3,3,3-hexamethyl-disilazane; n-butyllithium / tetrahydrofuran; hexane / 2 h / -78 - -40 °C / Inert atmosphere 2.2: 0.08 h / -78 °C / Inert atmosphere 3.1: hydrogenchloride / dichloromethane; ethanol; water / 0.5 h / 0 °C / Inert atmosphere 3.2: Inert atmosphere 4.1: pyridinium p-toluenesulfonate / N,N-dimethyl-formamide / 4 h / 0 - 20 °C / Inert atmosphere 4.2: Inert atmosphere 5.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 0 °C / Inert atmosphere 5.2: 20 °C / Inert atmosphere 6.1: pyridine; hexaammonium heptamolybdate tetrahydrate; dihydrogen peroxide / tert-butyl alcohol / 4 h / 0 - 20 °C / Inert atmosphere 7.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 12 h / 20 °C / Inert atmosphere 8.1: triphenylphosphine; iodine; 1H-imidazole / 0 °C / Inert atmosphere 9.1: sodium hexamethyldisilazane / tetrahydrofuran / 0.5 h / 0 °C / Inert atmosphere 10.1: magnesium / methanol; tetrahydrofuran / 20 °C / Inert atmosphere 11.1: pyridinium p-toluenesulfonate / methanol; ethylene glycol / 0.33 h / 20 °C / Inert atmosphere 12.1: manganese(IV) oxide / dichloromethane / 48 h / 20 °C / Inert atmosphere View Scheme |

-
-
553-21-9
costunolide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1.1: 1,1,1,3,3,3-hexamethyl-disilazane; n-butyllithium / tetrahydrofuran; hexane / 2 h / -78 - -40 °C / Inert atmosphere 1.2: 0.08 h / -78 °C / Inert atmosphere 2.1: hydrogenchloride / dichloromethane; ethanol; water / 0.5 h / 0 °C / Inert atmosphere 2.2: Inert atmosphere 3.1: pyridinium p-toluenesulfonate / N,N-dimethyl-formamide / 4 h / 0 - 20 °C / Inert atmosphere 3.2: Inert atmosphere 4.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 0 °C / Inert atmosphere 4.2: 20 °C / Inert atmosphere 5.1: pyridine; hexaammonium heptamolybdate tetrahydrate; dihydrogen peroxide / tert-butyl alcohol / 4 h / 0 - 20 °C / Inert atmosphere 6.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 12 h / 20 °C / Inert atmosphere 7.1: carbon tetrabromide; 2,6-dimethylpyridine; triphenylphosphine / dichloromethane / 0.5 h / 0 °C / Inert atmosphere 8.1: potassium hexamethylsilazane / tetrahydrofuran / 0.25 h / 0 °C / Inert atmosphere 9.1: magnesium / methanol; tetrahydrofuran / 20 °C / Inert atmosphere 10.1: pyridinium p-toluenesulfonate / methanol; ethylene glycol / 0.33 h / 20 °C / Inert atmosphere 11.1: manganese(IV) oxide / dichloromethane / 48 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 11 steps 1.1: 1,1,1,3,3,3-hexamethyl-disilazane; n-butyllithium / tetrahydrofuran; hexane / 2 h / -78 - -40 °C / Inert atmosphere 1.2: 0.08 h / -78 °C / Inert atmosphere 2.1: hydrogenchloride / dichloromethane; ethanol; water / 0.5 h / 0 °C / Inert atmosphere 2.2: Inert atmosphere 3.1: pyridinium p-toluenesulfonate / N,N-dimethyl-formamide / 4 h / 0 - 20 °C / Inert atmosphere 3.2: Inert atmosphere 4.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 0 °C / Inert atmosphere 4.2: 20 °C / Inert atmosphere 5.1: pyridine; hexaammonium heptamolybdate tetrahydrate; dihydrogen peroxide / tert-butyl alcohol / 4 h / 0 - 20 °C / Inert atmosphere 6.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 12 h / 20 °C / Inert atmosphere 7.1: triphenylphosphine; iodine; 1H-imidazole / 0 °C / Inert atmosphere 8.1: sodium hexamethyldisilazane / tetrahydrofuran / 0.5 h / 0 °C / Inert atmosphere 9.1: magnesium / methanol; tetrahydrofuran / 20 °C / Inert atmosphere 10.1: pyridinium p-toluenesulfonate / methanol; ethylene glycol / 0.33 h / 20 °C / Inert atmosphere 11.1: manganese(IV) oxide / dichloromethane / 48 h / 20 °C / Inert atmosphere View Scheme |
-
-
127969-90-8
(2E,6E)-8-((tert-butyldiphenylsilyl)oxy)-3,7-dimethylocta-2,6-dien-1-ol

-
-
553-21-9
costunolide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1.1: manganese(IV) oxide / dichloromethane / 6 h / Reflux; Inert atmosphere 1.2: Inert atmosphere 2.1: 1,1,1,3,3,3-hexamethyl-disilazane; n-butyllithium / tetrahydrofuran; hexane / 2 h / -78 - -40 °C / Inert atmosphere 2.2: 0.08 h / -78 °C / Inert atmosphere 3.1: hydrogenchloride / dichloromethane; ethanol; water / 0.5 h / 0 °C / Inert atmosphere 3.2: Inert atmosphere 4.1: pyridinium p-toluenesulfonate / N,N-dimethyl-formamide / 4 h / 0 - 20 °C / Inert atmosphere 4.2: Inert atmosphere 5.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 0 °C / Inert atmosphere 5.2: 20 °C / Inert atmosphere 6.1: pyridine; hexaammonium heptamolybdate tetrahydrate; dihydrogen peroxide / tert-butyl alcohol / 4 h / 0 - 20 °C / Inert atmosphere 7.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 12 h / 20 °C / Inert atmosphere 8.1: carbon tetrabromide; 2,6-dimethylpyridine; triphenylphosphine / dichloromethane / 0.5 h / 0 °C / Inert atmosphere 9.1: potassium hexamethylsilazane / tetrahydrofuran / 0.25 h / 0 °C / Inert atmosphere 10.1: magnesium / methanol; tetrahydrofuran / 20 °C / Inert atmosphere 11.1: pyridinium p-toluenesulfonate / methanol; ethylene glycol / 0.33 h / 20 °C / Inert atmosphere 12.1: manganese(IV) oxide / dichloromethane / 48 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 12 steps 1.1: manganese(IV) oxide / dichloromethane / 6 h / Reflux; Inert atmosphere 1.2: Inert atmosphere 2.1: 1,1,1,3,3,3-hexamethyl-disilazane; n-butyllithium / tetrahydrofuran; hexane / 2 h / -78 - -40 °C / Inert atmosphere 2.2: 0.08 h / -78 °C / Inert atmosphere 3.1: hydrogenchloride / dichloromethane; ethanol; water / 0.5 h / 0 °C / Inert atmosphere 3.2: Inert atmosphere 4.1: pyridinium p-toluenesulfonate / N,N-dimethyl-formamide / 4 h / 0 - 20 °C / Inert atmosphere 4.2: Inert atmosphere 5.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 0 °C / Inert atmosphere 5.2: 20 °C / Inert atmosphere 6.1: pyridine; hexaammonium heptamolybdate tetrahydrate; dihydrogen peroxide / tert-butyl alcohol / 4 h / 0 - 20 °C / Inert atmosphere 7.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 12 h / 20 °C / Inert atmosphere 8.1: triphenylphosphine; iodine; 1H-imidazole / 0 °C / Inert atmosphere 9.1: sodium hexamethyldisilazane / tetrahydrofuran / 0.5 h / 0 °C / Inert atmosphere 10.1: magnesium / methanol; tetrahydrofuran / 20 °C / Inert atmosphere 11.1: pyridinium p-toluenesulfonate / methanol; ethylene glycol / 0.33 h / 20 °C / Inert atmosphere 12.1: manganese(IV) oxide / dichloromethane / 48 h / 20 °C / Inert atmosphere View Scheme |

-
-
553-21-9
costunolide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: pyridinium p-toluenesulfonate / N,N-dimethyl-formamide / 4 h / 0 - 20 °C / Inert atmosphere 1.2: Inert atmosphere 2.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 0 °C / Inert atmosphere 2.2: 20 °C / Inert atmosphere 3.1: pyridine; hexaammonium heptamolybdate tetrahydrate; dihydrogen peroxide / tert-butyl alcohol / 4 h / 0 - 20 °C / Inert atmosphere 4.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 12 h / 20 °C / Inert atmosphere 5.1: carbon tetrabromide; 2,6-dimethylpyridine; triphenylphosphine / dichloromethane / 0.5 h / 0 °C / Inert atmosphere 6.1: potassium hexamethylsilazane / tetrahydrofuran / 0.25 h / 0 °C / Inert atmosphere 7.1: magnesium / methanol; tetrahydrofuran / 20 °C / Inert atmosphere 8.1: pyridinium p-toluenesulfonate / methanol; ethylene glycol / 0.33 h / 20 °C / Inert atmosphere 9.1: manganese(IV) oxide / dichloromethane / 48 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 9 steps 1.1: pyridinium p-toluenesulfonate / N,N-dimethyl-formamide / 4 h / 0 - 20 °C / Inert atmosphere 1.2: Inert atmosphere 2.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 0 °C / Inert atmosphere 2.2: 20 °C / Inert atmosphere 3.1: pyridine; hexaammonium heptamolybdate tetrahydrate; dihydrogen peroxide / tert-butyl alcohol / 4 h / 0 - 20 °C / Inert atmosphere 4.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 12 h / 20 °C / Inert atmosphere 5.1: triphenylphosphine; iodine; 1H-imidazole / 0 °C / Inert atmosphere 6.1: sodium hexamethyldisilazane / tetrahydrofuran / 0.5 h / 0 °C / Inert atmosphere 7.1: magnesium / methanol; tetrahydrofuran / 20 °C / Inert atmosphere 8.1: pyridinium p-toluenesulfonate / methanol; ethylene glycol / 0.33 h / 20 °C / Inert atmosphere 9.1: manganese(IV) oxide / dichloromethane / 48 h / 20 °C / Inert atmosphere View Scheme |

-
-
553-21-9
costunolide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: hydrogenchloride / dichloromethane; ethanol; water / 0.5 h / 0 °C / Inert atmosphere 1.2: Inert atmosphere 2.1: pyridinium p-toluenesulfonate / N,N-dimethyl-formamide / 4 h / 0 - 20 °C / Inert atmosphere 2.2: Inert atmosphere 3.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 0 °C / Inert atmosphere 3.2: 20 °C / Inert atmosphere 4.1: pyridine; hexaammonium heptamolybdate tetrahydrate; dihydrogen peroxide / tert-butyl alcohol / 4 h / 0 - 20 °C / Inert atmosphere 5.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 12 h / 20 °C / Inert atmosphere 6.1: carbon tetrabromide; 2,6-dimethylpyridine; triphenylphosphine / dichloromethane / 0.5 h / 0 °C / Inert atmosphere 7.1: potassium hexamethylsilazane / tetrahydrofuran / 0.25 h / 0 °C / Inert atmosphere 8.1: magnesium / methanol; tetrahydrofuran / 20 °C / Inert atmosphere 9.1: pyridinium p-toluenesulfonate / methanol; ethylene glycol / 0.33 h / 20 °C / Inert atmosphere 10.1: manganese(IV) oxide / dichloromethane / 48 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 10 steps 1.1: hydrogenchloride / dichloromethane; ethanol; water / 0.5 h / 0 °C / Inert atmosphere 1.2: Inert atmosphere 2.1: pyridinium p-toluenesulfonate / N,N-dimethyl-formamide / 4 h / 0 - 20 °C / Inert atmosphere 2.2: Inert atmosphere 3.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 0 °C / Inert atmosphere 3.2: 20 °C / Inert atmosphere 4.1: pyridine; hexaammonium heptamolybdate tetrahydrate; dihydrogen peroxide / tert-butyl alcohol / 4 h / 0 - 20 °C / Inert atmosphere 5.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 12 h / 20 °C / Inert atmosphere 6.1: triphenylphosphine; iodine; 1H-imidazole / 0 °C / Inert atmosphere 7.1: sodium hexamethyldisilazane / tetrahydrofuran / 0.5 h / 0 °C / Inert atmosphere 8.1: magnesium / methanol; tetrahydrofuran / 20 °C / Inert atmosphere 9.1: pyridinium p-toluenesulfonate / methanol; ethylene glycol / 0.33 h / 20 °C / Inert atmosphere 10.1: manganese(IV) oxide / dichloromethane / 48 h / 20 °C / Inert atmosphere View Scheme |

-
-
553-21-9
costunolide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 0 °C / Inert atmosphere 1.2: 20 °C / Inert atmosphere 2.1: pyridine; hexaammonium heptamolybdate tetrahydrate; dihydrogen peroxide / tert-butyl alcohol / 4 h / 0 - 20 °C / Inert atmosphere 3.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 12 h / 20 °C / Inert atmosphere 4.1: carbon tetrabromide; 2,6-dimethylpyridine; triphenylphosphine / dichloromethane / 0.5 h / 0 °C / Inert atmosphere 5.1: potassium hexamethylsilazane / tetrahydrofuran / 0.25 h / 0 °C / Inert atmosphere 6.1: magnesium / methanol; tetrahydrofuran / 20 °C / Inert atmosphere 7.1: pyridinium p-toluenesulfonate / methanol; ethylene glycol / 0.33 h / 20 °C / Inert atmosphere 8.1: manganese(IV) oxide / dichloromethane / 48 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 8 steps 1.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 0 °C / Inert atmosphere 1.2: 20 °C / Inert atmosphere 2.1: pyridine; hexaammonium heptamolybdate tetrahydrate; dihydrogen peroxide / tert-butyl alcohol / 4 h / 0 - 20 °C / Inert atmosphere 3.1: tetrabutyl ammonium fluoride / tetrahydrofuran / 12 h / 20 °C / Inert atmosphere 4.1: triphenylphosphine; iodine; 1H-imidazole / 0 °C / Inert atmosphere 5.1: sodium hexamethyldisilazane / tetrahydrofuran / 0.5 h / 0 °C / Inert atmosphere 6.1: magnesium / methanol; tetrahydrofuran / 20 °C / Inert atmosphere 7.1: pyridinium p-toluenesulfonate / methanol; ethylene glycol / 0.33 h / 20 °C / Inert atmosphere 8.1: manganese(IV) oxide / dichloromethane / 48 h / 20 °C / Inert atmosphere View Scheme |

-
-
553-21-9
costunolide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium hexamethyldisilazane / tetrahydrofuran / 0.5 h / 0 °C / Inert atmosphere 2: magnesium / methanol; tetrahydrofuran / 20 °C / Inert atmosphere 3: pyridinium p-toluenesulfonate / methanol; ethylene glycol / 0.33 h / 20 °C / Inert atmosphere 4: manganese(IV) oxide / dichloromethane / 48 h / 20 °C / Inert atmosphere View Scheme |

-
-
553-21-9
costunolide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: pyridine; hexaammonium heptamolybdate tetrahydrate; dihydrogen peroxide / tert-butyl alcohol / 4 h / 0 - 20 °C / Inert atmosphere 2: tetrabutyl ammonium fluoride / tetrahydrofuran / 12 h / 20 °C / Inert atmosphere 3: carbon tetrabromide; 2,6-dimethylpyridine; triphenylphosphine / dichloromethane / 0.5 h / 0 °C / Inert atmosphere 4: potassium hexamethylsilazane / tetrahydrofuran / 0.25 h / 0 °C / Inert atmosphere 5: magnesium / methanol; tetrahydrofuran / 20 °C / Inert atmosphere 6: pyridinium p-toluenesulfonate / methanol; ethylene glycol / 0.33 h / 20 °C / Inert atmosphere 7: manganese(IV) oxide / dichloromethane / 48 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 7 steps 1: pyridine; hexaammonium heptamolybdate tetrahydrate; dihydrogen peroxide / tert-butyl alcohol / 4 h / 0 - 20 °C / Inert atmosphere 2: tetrabutyl ammonium fluoride / tetrahydrofuran / 12 h / 20 °C / Inert atmosphere 3: triphenylphosphine; iodine; 1H-imidazole / 0 °C / Inert atmosphere 4: sodium hexamethyldisilazane / tetrahydrofuran / 0.5 h / 0 °C / Inert atmosphere 5: magnesium / methanol; tetrahydrofuran / 20 °C / Inert atmosphere 6: pyridinium p-toluenesulfonate / methanol; ethylene glycol / 0.33 h / 20 °C / Inert atmosphere 7: manganese(IV) oxide / dichloromethane / 48 h / 20 °C / Inert atmosphere View Scheme |

-
-
553-21-9
costunolide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: tetrabutyl ammonium fluoride / tetrahydrofuran / 12 h / 20 °C / Inert atmosphere 2: carbon tetrabromide; 2,6-dimethylpyridine; triphenylphosphine / dichloromethane / 0.5 h / 0 °C / Inert atmosphere 3: potassium hexamethylsilazane / tetrahydrofuran / 0.25 h / 0 °C / Inert atmosphere 4: magnesium / methanol; tetrahydrofuran / 20 °C / Inert atmosphere 5: pyridinium p-toluenesulfonate / methanol; ethylene glycol / 0.33 h / 20 °C / Inert atmosphere 6: manganese(IV) oxide / dichloromethane / 48 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 6 steps 1: tetrabutyl ammonium fluoride / tetrahydrofuran / 12 h / 20 °C / Inert atmosphere 2: triphenylphosphine; iodine; 1H-imidazole / 0 °C / Inert atmosphere 3: sodium hexamethyldisilazane / tetrahydrofuran / 0.5 h / 0 °C / Inert atmosphere 4: magnesium / methanol; tetrahydrofuran / 20 °C / Inert atmosphere 5: pyridinium p-toluenesulfonate / methanol; ethylene glycol / 0.33 h / 20 °C / Inert atmosphere 6: manganese(IV) oxide / dichloromethane / 48 h / 20 °C / Inert atmosphere View Scheme |

-
-
553-21-9
costunolide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: tert.-butyl lithium; N,N,N,N,-tetramethylethylenediamine / hexane; pentane / 36 h / 20 °C / Inert atmosphere 1.2: 3 h / -78 - -30 °C 2.1: manganese(IV) oxide / dichloromethane / 12 h / 20 °C View Scheme |
Costunolide Chemical Properties
Molecular Structure of Costunolide (CAS NO.553-21-9):
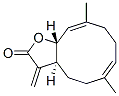
IUPAC: (3AS,6E,10E,11aR)-6,10-dimethyl-3-methylene-3a,4,5,8,9,11a-hexahydrocyclodeca[d]furan-2-one
Molecular Formula:C15H20O2
Molecular Weight:232.32
Product Categories:Miscellaneous Natural Products;chiral
Density:1.03 g/cm3
Flash Point:162oC
Boiling Point:385.4oC at 760 mmHg
SMILES:O=C/1O[C@@H]2/C=C(/CC/C=C(/CC[C@H]2C\1=C)C)C
InChI:InChI=1/C15H20O2/c1-10-5-4-6-11(2)9-14-13(8-7-10)12(3)15(16)17-14/h5,9,13-14H,3-4,6-8H2,1-2H3/b10-5+,11-9+/t13-,14+/m0/s1
InChIKey:HRYLQFBHBWLLLL-AHNJNIBGBA
Index of Refraction:1.519
Molar Volume:224.4 cm3
Surface Tension:35.1 dyne/cm
Molar Refractivity:68.12 cm3
Enthalpy of Vaporization:63.42 kJ/mol
Vapour Pressure:3.8E-06 mmHg at 25oC
Costunolide Production
Costunolide with cas registry number of 553-21-9 can be obtained from tree eremanthus elaegnus .
Costunolide Specification
Costunolide with cas registry number of 553-21-9 is also known as (3AS,6E,10E,11AR)-3A,4,5,8,9,11A-hexahydro-6,10-dimethyl-3-methylene-cyclodeca[B]furan-2(3H)-one ; Costunolide ; (E,E)-6-alpha-hydroxygermacra-1(10),4,11(13)-trien-12-oicacidgamma-lactone ; Costunolid ; Germacra-1(10),4,11(13)-trien-12-oicacid,6-alpha-hydroxy-,gamma-lactone,(e, ; Cyclodeca[b]furan-2(3H)-one,3a,4,5,8,9,11a-hexahydro-6,10-dimethyl-3- methylene-,[3aS-(3aR*,6E,10E,11aS*)]- ; Custunolide ; (3AS,6E,10E,11aR)-6,10-Dimethyl-3-methylidene-3a,4,5,8,9,11a-hexahydrocyclodeca[d]furan-2-one . Costunolide is an active sesquiterpene lactone of medicinal herbs with anti-inflammatory and potential anti-cancer activity. It affects nuclear organization and reorganized microtubule architecture. Costunolide demonstrates polymerizing ability, by inducing the formation of well organized microtubule polymers. It also used as chemoprophylactic agent in schistosomiasis.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View