-
Name
Cyclohexyltrichlorosilane
- EINECS
- CAS No. 98-12-4
- Article Data31
- CAS DataBase
- Density 1.2g/cm3
- Solubility
- Melting Point
- Formula C6H11 Cl3 Si
- Boiling Point 200.2°Cat760mmHg
- Molecular Weight 217.598
- Flash Point 87°C
- Transport Information
- Appearance CLEAR COLOURLESS LIQUID
- Safety A highly toxic and corrosive material. When heated to decomposition it emits toxic fumes of Cl−. See also CHLOROSILANES.
- Risk Codes 14-34
-
Molecular Structure
- Hazard Symbols Toxic by ingestion and inhalation, strong irritant to tissue.
- Synonyms Silane,trichlorocyclohexyl- (6CI,7CI,8CI,9CI); (Trichlorosilyl)cyclohexane;Cyclohexyltrichlorosilane; Trichlorocyclohexylsilane
- PSA 0.00000
- LogP 3.97600
Cyclohexyltrichlorosilane Chemical Properties
IUPAC Name: Trichloro(cyclohexyl)silane
Synonyms of Cyclohexyltrichlorosilane (CAS NO.98-12-4): Cyclohexane, (trichlorosilyl)- ; Silane, trichlorocyclohexyl- ; Trichloro(cyclohexyl)silane
Product Categories: Functional Materials ;Si (Classes of Silicon Compounds);Si-Cl Compounds;Silane Coupling Agents;Silane Coupling Agents (Intermediates);Trichlorosilanes
Product Categories: Inorganic Fluorides
CAS NO: 98-12-4
Molecular Formula: C6H11Cl3Si
Molecular Weight : 217.6
Molecular Structure: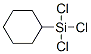
EINECS: 202-639-2
H bond acceptors: 0
H bond donors: 0
Freely Rotating Bonds: 1
Polar Surface Area: 0 Å2
Index of Refraction: 1.477
Molar Refractivity: 51.01 cm3
Molar Volume: 180.3 cm3
Surface Tension: 28.1 dyne/cm
Density: 1.2 g/cm3
Flash Point: 87 °C
Enthalpy of Vaporization: 41.86 kJ/mol
Boiling Point: 200.2 °C at 760 mmHg
Vapour Pressure: 0.465 mmHg at 25°C
Sensitive: Moisture Sensitive
Appearance: Cyclohexyltrichlorosilane (CAS NO.98-12-4) is a colorless to pale yellow liquid with a pungent odor.
Cyclohexyltrichlorosilane Consensus Reports
Reported in EPA TSCA Inventory.
Cyclohexyltrichlorosilane Safety Profile
Hazard Codes:  C
C
Risk Statements: 14-34
R14: Reacts violently with water.
R34: Cyclohexyltrichlorosilane (CAS NO.98-12-4) causes burns.
Safety Statements: 26-28-36/37/39-45
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S28: After contact with skin, wash immediately with plenty of soap-suds.
S36/37/39: Wear suitable protective clothing, gloves and eye/face protection.
S45: In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
RIDADR: UN 1763 8/PG 2
WGK Germany: 3
RTECS: VV2890000
F: 10-21
TSCA: Yes
HazardClass: 8
PackingGroup: II
A highly toxic and corrosive material. When heated to decomposition it emits toxic fumes of Cl−. See also CHLOROSILANES.
Cyclohexyltrichlorosilane Standards and Recommendations
DOT Classification: 8; Label: Corrosive
Cyclohexyltrichlorosilane Specification
1.Reactivity Profile: Chlorosilanes, such as Cyclohexyltrichlorosilane, are compounds in which silicon is bonded to from one to four chlorine atoms with other bonds to hydrogen and/or alkyl groups. Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. They may also produce flammable gaseous H2. They can serve as chlorination agents. Chlorosilanes react vigorously with both organic and inorganic acids and with bases to generate toxic or flammable gases.
2.Health Hazard: TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Contact with molten substance may cause severe burns to skin and eyes. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
3.Fire Hazard: Combustible material: may burn but does not ignite readily. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Related Products
- Cyclohexyltrichlorosilane
- 98130-56-4
- 98130-58-6
- 981-34-0
- 98134-36-2
- 98-13-5
- 98136-86-8
- 98138-05-7
- 98139-15-2
- 98140-96-6
- 98141-15-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View