-
Name
CYCLOPROPANE
- EINECS 200-847-8
- CAS No. 75-19-4
- Article Data172
- CAS DataBase
- Density 0.79 g/cm3
- Solubility
- Melting Point -128 °C(lit.)
- Formula C3H6
- Boiling Point −33 °C(lit.)
- Molecular Weight 42.0806
- Flash Point
- Transport Information
- Appearance
- Safety 9-16-33
- Risk Codes 12
-
Molecular Structure
-
Hazard Symbols
 F+
F+
- Synonyms 151821-32-8;Cyclopropane ring;Cyclopropanum [INN-Latin];Cyclopropane [UN1027] [Flammable gas];Trimethylene (cyclic);Cyclopropane [Anaesthetics, volatile];Ciclopropano [INN-Spanish];Trimethylene;30000-70-5;RC 270;1/C3H6/c1-2-3-1/h1-3H;Cyclopropane [INN];CYCLOPROPANE, liquefied (DOT);
- PSA 0.00000
- LogP 1.17030
Cyclopropane History
Cyclopropane (CAS No.:75-19-4) was discovered in 1881 by August Freund, who also proposed the right structure for the new substance in his first paper. Freund reacted 1,3-dibromopropane with sodium, the reaction is an intramolecular Wurtz reaction leading directly to cyclopropane.The yield of the reaction can be improved by the use of zinc instead of sodium.Cyclopropane had no commercial application until Henderson and Lucas discovered its anaesthetic properties in 1929 ; industrial production had begun by 1936.
Cyclopropane Consensus Reports
IARC Cancer Review: Animal No Adequate Data IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 7 , 1987,p. 93.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . Reported in EPA TSCA Inventory.
Cyclopropane Standards and Recommendations
DOT Classification: 2.1; Label: Flammable Gas
Cyclopropane Specification
The Cyclopropane is an organic compound with the formula C3H6. The IUPAC name of this chemical is cyclopropane. With the CAS registry number 75-19-4, it is also named as Cyclopropan. The product's categories are Organics; Chemical Synthesis; Compressed and Liquefied Gases; Synthetic Reagents. Besides, it should be stored in a closed and dry place.
The Cyclopropane is an anaesthetic when inhaled. In modern anaesthetic practice, it has been superseded by other agents, due to its extreme reactivity under normal conditions: when the gas is mixed with oxygen there is a significant risk of explosion.
Physical properties about Cyclopropane are: (1)ACD/LogP: 1.58; (2)ACD/LogD (pH 5.5): 1.581; (3)ACD/LogD (pH 7.4): 1.581; (4)ACD/BCF (pH 5.5): 9.371; (5)ACD/BCF (pH 7.4): 9.371; (6)ACD/KOC (pH 5.5): 172.668; (7)ACD/KOC (pH 7.4): 172.668; (8)Index of Refraction: 1.433; (9)Molar Refractivity: 13.835 cm3; (10)Molar Volume: 53.24 cm3; (11)Polarizability: 5.485×10-24cm3; (12)Surface Tension: 25.926 dyne/cm; (13)Density: 0.79 g/cm3; (14)Enthalpy of Vaporization: 20.05 kJ/mol; (15)Vapour Pressure: 5348.461 mmHg at 25°C.
Preparation: this chemical can be prepared by 1,3-dibromo-propane. This reaction will need reagent zinc dust. The reaction temperature is 200 - 300 °C. The yield is about 78%.
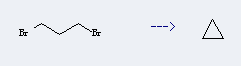
Uses of Cyclopropane: it can be used to produce 1,3-dichloro-propane, 3-chloro-propene, chloro-cyclopropane, 1,1-dichloro-cyclopropane at temperature of 600 °C. It will need reagent Cl2, SiF4. The yield is about 83.5%.

When you are using this chemical, please be cautious about it as the following:
It is extremely flammable. Plaese keep container in a well-ventilated place. When you are using it, take precautionary measures against static discharges and keep away from sources of ignition - No smoking.
You can still convert the following datas into molecular structure:
(1)SMILES: C1CC1
(2)InChI: InChI=1/C3H6/c1-2-3-1/h1-3H2
(3)InChIKey: LVZWSLJZHVFIQJ-UHFFFAOYAL
(4)Std. InChI: InChI=1S/C3H6/c1-2-3-1/h1-3H2
(5)Std. InChIKey: LVZWSLJZHVFIQJ-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LCLo | inhalation | 282gm/m3/2H (282000mg/m3) | BEHAVIORAL: EXCITEMENT | Journal of Pharmacology and Experimental Therapeutics. Vol. 58, Pg. 74, 1936. |
Related Products
- Cyclopropane
- Cyclopropane, bromo-, radical ion(1+)
- Cyclopropane, isocyanato-
- Cyclopropane, pentachloro-
- Cyclopropane,(iodomethyl)-
- Cyclopropane,[2-(trimethylsilyl)ethynyl]-
- Cyclopropane,1,1-dichloro-2-(3-methylbutoxy)-
- Cyclopropane,1,1-diethyl-
- Cyclopropane,1,1'-methylenebis-
- Cyclopropane,2-ethenyl-1,1-difluoro-
- 751-94-0
- 75195-73-2
- 7520-01-6
- 75203-51-9
- 7520-69-6
- 75-20-7
- 75207-72-6
- 75213-31-9
- 75214-12-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View