-
Name
ADONITOL
- EINECS 207-685-7
- CAS No. 488-81-3
- Article Data56
- CAS DataBase
- Density 1.526 g/cm3
- Solubility Soluble in water (100 mg/ml).
- Melting Point 102-105 °C
- Formula C5H12O5
- Boiling Point 494.527 °C at 760 mmHg
- Molecular Weight 152.147
- Flash Point 261.85 °C
- Transport Information
- Appearance White powder
- Safety 24/25-36-26
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Adonitol(7CI);Adonit;Adonite;NSC 16868;ribo-Pentitol;Ribitol;
- PSA 101.15000
- LogP -2.94630
Synthetic route

-
-
488-81-3
Adonitol

| Conditions | Yield |
|---|---|
| Stage #1: C17H42O5Si4 With bis(pentafluorophenyl)borinic acid; 1,1,3,3-tetramethyldisilazane In 1,4-dioxane at 25℃; for 24h; Inert atmosphere; Glovebox; Stage #2: In methanol Inert atmosphere; Glovebox; chemoselective reaction; | 92% |
-
-
532-20-7, 613-83-2, 7261-25-8, 7687-39-0, 13221-22-2, 14795-83-6, 15761-67-8, 20074-49-1, 25545-03-3, 25545-04-4, 32445-75-3, 34436-17-4, 36441-93-7, 36468-53-8, 37110-85-3, 37388-49-1, 38029-69-5, 40461-77-6, 40461-89-0, 41546-19-4, 41546-20-7, 41546-21-8, 41546-26-3, 41546-29-6, 41546-30-9, 41546-31-0, 126872-16-0, 131064-98-7
D-Ribose

-
-
488-81-3
Adonitol

| Conditions | Yield |
|---|---|
| With methanol; lithium borohydride at 10 - 20℃; for 3h; Large scale; | 78% |
-
-
54-17-1, 492-61-5, 492-62-6, 492-66-0, 530-25-6, 530-26-7, 530-27-8, 530-28-9, 552-76-1, 579-36-2, 643-69-6, 655-45-8, 2280-44-6, 3646-73-9, 7282-78-2, 7282-79-3, 7282-80-6, 7282-81-7, 7282-82-8, 7283-02-5, 7283-08-1, 7283-09-2, 7283-10-5, 7283-11-6, 7296-15-3, 7296-64-2, 7322-31-8, 10257-28-0, 12772-65-5, 12773-29-4, 12773-31-8, 12773-32-9, 12773-33-0, 12773-34-1, 19982-85-5, 28538-24-1, 32445-46-8, 32445-47-9, 32445-51-5, 32445-52-6, 34685-56-8, 34685-57-9, 35810-56-1, 39281-65-7, 39281-66-8, 39281-67-9, 39281-68-0, 39281-69-1, 39392-61-5, 39392-62-6, 39392-63-7, 39392-65-9, 39392-66-0, 39392-69-3, 40825-84-1, 40825-86-3, 40825-87-4, 40825-89-6, 42752-07-8, 42789-83-3, 46032-76-2, 54808-89-8, 66069-07-6, 120442-57-1, 120442-58-2, 120442-59-3, 120442-67-3, 131064-91-0, 131064-92-1, 131065-00-4, 131065-01-5, 131348-38-4
D-allose

-
-
488-81-3
Adonitol

| Conditions | Yield |
|---|---|
| With 1-methyl-pyrrolidin-2-one; Wilkinson's catalyst at 130℃; | 50% |
-
-
18524-23-7, 876594-33-1
DL-erythro-pentene-(1)-triol-(3.4.5)-triacetate

-
A
-
488-81-3
Adonitol

-
-
5346-78-1, 5401-55-8, 6330-69-4, 7208-42-6, 26674-23-7, 74033-71-9, 85761-13-3
1,2,3,4,5-penta-O-acetyl-DL-arabinitol

| Conditions | Yield |
|---|---|
| With osmium(VIII) oxide; silver(I) chlorate und Acetylierung des erhaltenen Gemisches von Ribit-1.2.3-triacetat und D-Arabit-3.4.5-triacetat Verseifen des Pentaacetats mit methylalkoh.HCl; |

| Conditions | Yield |
|---|---|
| With sodium metaborate; water | |
| With nickel; magnesium at 100℃; under 22065.2 Torr; Hydrogenation; | |
| Yield given. Multistep reaction; |
-
-
24259-59-4
L-ribose

-
-
488-81-3
Adonitol

| Conditions | Yield |
|---|---|
| With sodium amalgam |

-
-
488-81-3
Adonitol

| Conditions | Yield |
|---|---|
| With potassium acetate; acetic anhydride; acetic acid und Verseifen des entstandenen Pentaacetats mit siedender methylalkoh.HCl; |
-
-
57-48-7
D-Fructose

-
A
-
488-81-3
Adonitol

-
B
-
81893-52-9
(S)-3,4-dihydroxy-butyraldehyde

-
C
-
81539-41-5
2,3-dideoxy-3-C-hydroxymethyltetrose

-
D
-
81539-40-4, 132215-58-8
2-deoxy-2-C-hydroxymethyltetrose

-
E
-
107-21-1
ethylene glycol

-
F
-
583-50-6
D-erythrose

| Conditions | Yield |
|---|---|
| In water Quantum yield; Irradiation; 254-nm photolysis in deoxigenated and oxygenated solutions; |


| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate |
-
-
3445-24-7
D-erythro-pentos-2-ulose

-
A
-
488-82-4
D-Arabitol

-
B
-
488-81-3
Adonitol

-
C
-
488-84-6
D-ribulose

-
D
-
50-69-1
D-ribose

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In water at 80℃; for 0.5h; | A n/a B n/a C 70 % Chromat. D n/a |


| Conditions | Yield |
|---|---|
| With 4-nitroperbenzoic acid In dichloromethane at 90℃; |

-
-
81076-13-3
4,5-O-isopropylidene-D-ribitol

-
-
488-81-3
Adonitol

| Conditions | Yield |
|---|---|
| With Dowex 50X8-200 resin In water at 25℃; for 3h; | |
| With hydrogenchloride |
-
-
85716-54-7
(Z)-(4RS)-1,4,5-triacetoxypent-2-ene

-
-
488-81-3
Adonitol

| Conditions | Yield |
|---|---|
| With pyridine; hydrogenchloride; tert.-butylhydroperoxide; osmium(VIII) oxide; tetraethylammonium acetate; acetic anhydride Yield given. Multistep reaction; |
-
-
81028-11-7, 81076-13-3, 81076-14-4, 81076-15-5, 92935-75-6, 92935-76-7, 92935-77-8, 92935-78-9
(1R,2R)-1-((S)-2,2-Dimethyl-[1,3]dioxolan-4-yl)-propane-1,2,3-triol

-
-
488-81-3
Adonitol

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol Heating; | |
| With hydrogenchloride In methanol Heating; |
-
-
88367-64-0, 88391-55-3, 88391-65-5, 88391-66-6
(1R,2R)-(S)-1-(1,4-Dioxa-spiro[4.5]dec-2-yl)-propane-1,2,3-triol

-
-
488-81-3
Adonitol

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol Heating; | |
| With hydrogenchloride In methanol Heating; |
-
-
88367-62-8, 88391-53-1, 88391-61-1, 88391-62-2
2,2-Dimethyl-propionic acid (2R,3R)-3-((S)-2,2-dimethyl-[1,3]dioxolan-4-yl)-2,3-dihydroxy-propyl ester

-
-
488-81-3
Adonitol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In diethyl ether at 0℃; | |
| Multi-step reaction with 2 steps 1: 5 percent conc HCl / methanol / Heating 2: LiAlH4 / diethyl ether / 0 °C View Scheme |
-
-
88367-65-1, 88391-56-4, 88391-67-7, 88391-68-8
(2R,3R,4S)-4,5-Bis-benzyloxy-pentane-1,2,3-triol

-
-
488-81-3
Adonitol

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol; acetic acid | |
| With hydrogen; palladium on activated charcoal In methanol; acetic acid Ambient temperature; |
-
-
88367-63-9, 88391-54-2, 88391-63-3, 88391-64-4
(1S,2S)-3-(tert-Butyl-diphenyl-silanyloxy)-1-((R)-2,2-dimethyl-[1,3]dioxolan-4-yl)-propane-1,2-diol

-
-
488-81-3
Adonitol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In diethyl ether at 0℃; | |
| Multi-step reaction with 2 steps 1: 5 percent conc HCl / methanol / Heating 2: (n-Bu)4N(+)F(-) / tetrahydrofuran / Ambient temperature View Scheme |

-
-
488-81-3
Adonitol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In diethyl ether at 0℃; |

-
-
488-81-3
Adonitol

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran Ambient temperature; |

-
A
-
488-81-3
Adonitol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water |

-
-
226089-02-7
(2S,3S,4R)-5-((2R,3R,4S,5S,6R)-3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-pyran-2-yloxy)-pentane-1,2,3,4-tetraol

-
A
-
2280-44-6
D-Glucose

-
B
-
488-81-3
Adonitol

| Conditions | Yield |
|---|---|
| With sulfuric acid In 1,4-dioxane; water |
-
-
35320-17-3
D-ribitol 5-phosphate

-
-
488-81-3
Adonitol

| Conditions | Yield |
|---|---|
| Hydrolysis; |
-
-
488-30-2
arabinoic acid

-
A
-
909878-64-4
meso-erythritol

-
B
-
488-82-4
D-Arabitol

-
C
-
87-99-0
XYLITOL

-
D
-
488-81-3
Adonitol

| Conditions | Yield |
|---|---|
| With hydrogen; Ru-carbon In water at 80℃; under 45003.6 Torr; Product distribution; Further Variations:; Catalysts; pH-values; Temperatures; Reagents; |

-
-
141-46-8
Glycolaldehyde

-
-
56-82-6
Glyceraldehyde

-
A
-
2418-52-2
D-threitol

-
B
-
488-82-4
D-Arabitol

-
C
-
87-99-0
XYLITOL

-
D
-
488-81-3
Adonitol

| Conditions | Yield |
|---|---|
| Stage #1: Glycolaldehyde; Glyceraldehyde With zinc(II) bis(L-proline) In water at 20℃; for 168h; Stage #2: With sodium tetrahydroborate for 3h; Further byproducts given. Title compound not separated from byproducts; |


-
B
-
488-81-3
Adonitol

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 120℃; for 2h; |

-
-
488-81-3
Adonitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NaOMe / methanol 2: 5percent H2SO4 / H2O; dioxane View Scheme |
-
-
102419-56-7
(1R,4'R)-1-(2,2-dimethyl-1,3-dioxolane-4-yl)-2-trimethylsilylprop-2-en-1-ol

-
-
488-81-3
Adonitol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 85 percent / TBHP-VO(acac)2 2: 80 percent / t-BuOK, n-Bu4NF 3: 70 percent / NaOH / 70 °C 4: HCl View Scheme |
-
-
488-81-3
Adonitol

-
-
77-76-9
2,2-dimethoxy-propane

-
-
3969-85-5, 73543-86-9, 88390-55-0
bis(2,2-dimethyl-1,3-dioxolan-4-yl)methanol

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In N,N-dimethyl-formamide Ambient temperature; | 99% |
| With hydrogen cation In acetone for 2h; Ambient temperature; | 95% |
| With toluene-4-sulfonic acid In N,N-dimethyl-formamide; acetone at 20℃; for 16h; Cyclization; | 85% |

| Conditions | Yield |
|---|---|
| With 1-methyl-2,3,4,6,7,8-hexahydro-1H-pyrimido[1,2-a]pyrimidine In N,N-dimethyl-formamide at 25℃; for 24h; | 99% |

| Conditions | Yield |
|---|---|
| With Sulfuric acid immobilized on silica gel at 20℃; for 0.333333h; neat (no solvent); | 98% |
| With zinc(II) chloride anfangs unter Eiskuehlung; | |
| With pyridine Ambient temperature; |
-
-
488-81-3
Adonitol

| Conditions | Yield |
|---|---|
| With 1H-imidazole; thionyl chloride In tetrahydrofuran at -10℃; for 0.5h; | 98% |
-
-
488-81-3
Adonitol

-
-
849585-22-4
LACTIC ACID

| Conditions | Yield |
|---|---|
| With barium hydroxide octahydrate In methanol at 140℃; for 12h; Inert atmosphere; Sealed tube; | 93% |
| With H2O*Ba8H16O16 In methanol at 140℃; for 12h; Sealed tube; | 88 %Chromat. |

| Conditions | Yield |
|---|---|
| With potassium fluoride In dimethyl sulfoxide 1) 60 deg C, 10 h; 2) 90 deg C, 10 h; | 91% |
-
-
488-81-3
Adonitol

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 100℃; for 48h; | 90% |
| With pyridine hydrochloride at 150℃; | 70% |
| at 290℃; for 2h; | 45% |
| With hydrogenchloride at 110℃; |
-
-
488-81-3
Adonitol

| Conditions | Yield |
|---|---|
| With carbon tetrabromide; triphenylphosphine In N,N-dimethyl-formamide Ambient temperature; | 90% |
| In water at 249.84℃; Kinetics; Temperature; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 1.5h; Cooling with ice; | 88% |

| Conditions | Yield |
|---|---|
| With pyridine at 100℃; for 22h; Inert atmosphere; | 85% |
| With pyridine at 100℃; | |
| With pyridine at 20℃; | |
| With pyridine; dmap at 25℃; for 33h; | |
| With pyridine; dmap at 20℃; for 30h; Inert atmosphere; |
-
-
488-81-3
Adonitol

-
-
67-64-1
acetone

-
-
3969-86-6, 19139-74-3, 30737-85-0, 84709-27-3, 84709-35-3, 84709-41-1, 100484-23-9, 127305-49-1
1,2:3,4-Di-O-isopropylidene-DL-ribitol

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In various solvent(s) Ambient temperature; | 85% |
-
-
488-81-3
Adonitol

-
-
33842-02-3, 529510-96-1
dichloromethylenedimethyliminium chloride

-
-
143094-04-6, 143167-34-4, 143167-35-5
1,5-dichloro-1,5-dideoxy-2,4-di-O--ribitol

| Conditions | Yield |
|---|---|
| In 1,4-dioxane at 25℃; for 2.5h; | 79% |
| In 1,4-dioxane at 23℃; for 2.5h; | 79% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol; water for 8h; | 70% |

| Conditions | Yield |
|---|---|
| Stage #1: Adonitol With di(n-butyl)tin oxide In toluene Stage #2: phenylcarbonochloridothioate In chloroform at 20℃; for 4h; | 63% |
| Stage #1: Adonitol With di(n-butyl)tin oxide In toluene Heating; Stage #2: phenylcarbonochloridothioate In chloroform at 20℃; for 4h; | 63% |

| Conditions | Yield |
|---|---|
| Stage #1: Adonitol With pyridine hydrochloride at 150℃; for 4h; Stage #2: 2,2-dimethoxy-propane With toluene-4-sulfonic acid In acetone at 20℃; for 0.5h; Stage #3: C22H22ClF3N2O4S Further stages; | 58% |

-
-
488-81-3
Adonitol

-
-
100664-93-5
ribitol 3-O-(dichlorophosphite) 1,2;4,5-bis-O-(chlorophosphite)

| Conditions | Yield |
|---|---|
| With phosphorus trichloride In 1,4-dioxane at 60 - 65℃; for 30h; | 57% |
-
-
488-81-3
Adonitol

-
-
108-24-7
acetic anhydride

-
-
100-39-0
benzyl bromide

-
A
-
7208-42-6
penta-O-acetylribitol

| Conditions | Yield |
|---|---|
| Stage #1: Adonitol With bis(tri-n-butyltin)oxide In toluene for 5h; Heating; Stage #2: benzyl bromide at 80℃; for 20h; Stage #3: acetic anhydride With pyridine at 20℃; | A 23% B 52% C 16% |

-
-
488-81-3
Adonitol

-
-
33842-02-3, 529510-96-1
dichloromethylenedimethyliminium chloride

-
A
-
143094-04-6, 143167-34-4, 143167-35-5
1,5-dichloro-1,5-dideoxy-2,4-di-O--ribitol

| Conditions | Yield |
|---|---|
| In 1,4-dioxane at 23℃; for 26h; | A 47% B 20% |

-
-
488-81-3
Adonitol

-
-
33842-02-3, 529510-96-1
dichloromethylenedimethyliminium chloride

-
A
-
143094-04-6, 143167-34-4, 143167-35-5
1,5-dichloro-1,5-dideoxy-2,4-di-O--ribitol

| Conditions | Yield |
|---|---|
| In 1,4-dioxane at 23℃; for 26h; | A 47% B 21% |

-
-
488-81-3
Adonitol

-
-
108-24-7
acetic anhydride

-
-
100-39-0
benzyl bromide

-
A
-
7208-42-6
penta-O-acetylribitol

| Conditions | Yield |
|---|---|
| Stage #1: Adonitol With di(n-butyl)tin oxide In toluene for 16h; Heating; Stage #2: benzyl bromide In chloroform at 65℃; for 65h; Stage #3: acetic anhydride With pyridine at 20℃; | A 47% B 10% C n/a D n/a |
| Stage #1: Adonitol With di(n-butyl)tin oxide In toluene for 16h; Heating; Stage #2: benzyl bromide In chloroform at 50℃; for 40h; Stage #3: acetic anhydride With pyridine at 20℃; | A 32% B 31% C 8% D 17% |
| Stage #1: Adonitol With di(n-butyl)tin oxide In toluene for 16h; Heating; Stage #2: benzyl bromide In chloroform at 65℃; for 65h; Stage #3: acetic anhydride With pyridine at 20℃; | A 16% B 26% C 11% D 20% |


| Conditions | Yield |
|---|---|
| With (1S)-10-camphorsulfonic acid; phenylboronic acid In toluene at 120 - 130℃; for 24h; Inert atmosphere; Dean-Stark; | 47% |

| Conditions | Yield |
|---|---|
| With (1S)-10-camphorsulfonic acid; phenylboronic acid In toluene at 120 - 130℃; for 24h; Inert atmosphere; Dean-Stark; | 44% |

| Conditions | Yield |
|---|---|
| With C13H21O3Re at 135℃; for 15h; Reagent/catalyst; Inert atmosphere; | 42% |
-
-
488-81-3
Adonitol

-
-
122-52-1
triethyl phosphite

-
-
117562-69-3
3-O-(diethyl phosphite)-1,2:4,5-bis-O-(ethyl phosphite)ribitol

| Conditions | Yield |
|---|---|
| at 115 - 130℃; | 38% |
-
-
488-81-3
Adonitol

-
-
116-11-0
2-Methoxypropene

-
A
-
3969-85-5, 73543-86-9, 88390-55-0
bis(2,2-dimethyl-1,3-dioxolan-4-yl)methanol

-
-
100423-71-0, 100484-25-1
1,4:2,3-di-O-isopropylidene-DL-ribitol

-
-
131247-52-4
1,3:4,5-di-O-isopropylidene-DL-ribitol

-
-
131247-54-6
1,3:2,5-di-O-isopropylidene-DL-ribitol

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In N,N-dimethyl-formamide at 0℃; for 0.0166667h; | A 36% B 3% C 12% D 5% |
| With toluene-4-sulfonic acid In N,N-dimethyl-formamide at 0℃; for 0.0166667h; Product distribution; | A 36% B 3% C 12% D 5% |


| Conditions | Yield |
|---|---|
| With methyltrioxorhenium(VII) at 170℃; for 3h; | 33% |
-
-
488-81-3
Adonitol

-
-
120878-42-4
3,4-bis(decyloxy)benzoic acid

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane at 20℃; for 72h; Esterification; | 8% |
D-(+)-Arabitol Chemical Properties
Molecular Formula: C5H12O5
Molar mass: 152.15 g/mol
EINECS: 207-685-7
Density: 1.525 g/cm3
Flash Point: 261.9 °C
Index of Refraction: 1.57
Boiling Point: 494.5 °C at 760 mmHg
Vapour Pressure: 7.47E-12 mmHg at 25°C
Melting point: 102-105 °C
Storage temp: Store at RT.
Sensitive: Hygroscopic
Appearance: White powder
Product categories: Sugars, Carbohydrates & Glucosides;Biochemistry;Sugar Alcohols
Structure of Ribitol (488-81-3):
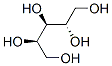
XLogP3-AA: -2.5
H-Bond Donor: 5
H-Bond Acceptor: 5
Systematic Name: Pentane-1,2,3,4,5-pentol
SMILES: OC(CO)C(O)C(O)CO
InChI: InChI=1/C5H12O5/c6-1-3(8)5(10)4(9)2-7/h3-10H,1-2H2
InChIKey: HEBKCHPVOIAQTA-UHFFFAOYAB
Std. InChI: InChI=1S/C5H12O5/c6-1-3(8)5(10)4(9)2-7/h3-10H,1-2H2
Std. InChIKey: HEBKCHPVOIAQTA-UHFFFAOYSA-N
D-(+)-Arabitol Production
Ribitol (488-81-3) is formed by the reduction of Ribose . It occurs naturally in the plant Adonis vernalis, as well as in the cell walls of Gram positive bacteria.
D-(+)-Arabitol Toxicity Data With Reference
| 1. | ipr-mus LDLo:10 g/kg | PSEBAA Proceedings of the Society for Experimental Biology and Medicine. 35 (1936),98. |
Carcinogenicity of Ribitol (488-81-3) hasn't been listed as a carcinogen by NTP, IARC,ACGIH, or CA Prop 65.You can see actual entry in RTECS for complete information.
D-(+)-Arabitol Consensus Reports
Reported in EPA TSCA Inventory.
D-(+)-Arabitol Safety Profile
Mildly toxic by intraperitoneal route. When heated to decomposition it emits acrid smoke and irritating fumes.
Hazard Codes: Xi![]()
Risk Statements:
36: Irritating to the eyes
37: Irritating to the respiratory system
38: Irritating to the skin
Safety Statements:
24: Avoid contact with skin
25: Avoid contact with eyes
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
D-(+)-Arabitol Specification
Ribitol (488-81-3) is a crystalline pentose alcohol, and also can be called adonitol ; pentitol ; Pentane-1,2,3,4,5-pentaol ; 1,2,3,4,5-Pentanepentol ; 1,2,3,4,5-Pentahydroxypentane .It is hazardous,so the first aid measures and others should be known.Such as: When on the skin: should flush skin with plenty of water immediatelyfor at least 15 minutes while removing contaminated clothing.Or in the eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids.While ,it's Inhaled: Remove from exposure and move to fresh air immediately.Then you have the ingesting of the product : Do not induce vomiting.If victim is conscious and alert, give 2-4 cupfuls of milk or water.
In addition, Ribitol (488-81-3) can be stable under normal temperature and pressure conditions.It is not compatible with strong oxidizing agents, and you must not take it with strong oxidants and incompatible materials.And also prevent it to broken down into hazardous decomposition products: irritating and toxic fumes and gases, carbon dioxide, carbon monoxide.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View