-
Name
Diethyl maleate
- EINECS 205-451-9
- CAS No. 141-05-9
- Article Data201
- CAS DataBase
- Density 1.068 g/cm3
- Solubility insoluble in water
- Melting Point -10 °C
- Formula C8H12O4
- Boiling Point 214 °C at 760 mmHg
- Molecular Weight 172.181
- Flash Point 93.3 °C
- Transport Information
- Appearance colourless liquid
- Safety 26-36/37-37-24
- Risk Codes 36-43
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2-Butenedioicacid (2Z)-, diethyl ester (9CI);2-Butenedioic acid (Z)-, diethyl ester;Maleicacid, diethyl ester (6CI,8CI);(2Z)-2-Butenedioic acid diethyl ester;Diethyl(Z)-2-butenedioate;2-Butenedioicacid (2Z)-, 1,4-diethyl ester;Ethyl maleate;Staflex DEM;
- PSA 52.60000
- LogP 0.66880
Synthetic route

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 20h; | 100% |
-
-
327-62-8
potassium propionate

-
-
99967-60-9
2-(Methylsulfonyloxy)-diethylester

-
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 20h; | 100% |
-
-
608-82-2, 1114-30-3, 1114-31-4, 40205-59-2
diethyl dl-dibromosuccinate

-
A
-
623-91-6
diethyl Fumarate

-
B
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| With N-benzyl-1,4-dihydronicotinamide In acetonitrile at 49.9℃; for 7h; | A 100% B n/a |

| Conditions | Yield |
|---|---|
| With trans-IICl2(pyboxip)(=CHSiMe3)> Ambient temperature; | 98% |
| tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride In dichloromethane at 20℃; for 15h; | 95% |
| tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride In dichloromethane at 20℃; | 95% |
-
-
623-73-4
diazoacetic acid ethyl ester

-
-
104575-54-4
N-(2-naphthalenylmethylene)butane-1-amine

-
B
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| methyltrioxorhenium(VII) | A 96% B n/a |

-
-
623-73-4
diazoacetic acid ethyl ester

-
-
172367-39-4
N-(2-naphthalenylmethylene)hexane-1-amine

-
B
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| methyltrioxorhenium(VII) | A 96% B n/a |

-
-
623-73-4
diazoacetic acid ethyl ester

-
-
627463-17-6
4-bromo-2-fluoro-1-vinylbenzene

-
A
-
934995-82-1
C12H12BrFO2

-
C
-
623-91-6
diethyl Fumarate

-
D
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| With aluminum oxide; potassium carbonate; copper(I) trifluoromethanesulfonate benzene; 2,2'-isopropylidenebis[(4S)-4-tert-butyl-2-oxazoline] In chloroform at 0 - 20℃; for 24 - 25h; Product distribution / selectivity; | A n/a B 96% C n/a D n/a |
| copper(I) trifluoromethanesulfonate benzene; 2,2'-isopropylidenebis[(4S)-4-tert-butyl-2-oxazoline] In ethyl acetate at 0 - 20℃; for 24 - 25h; Product distribution / selectivity; | A n/a B n/a C n/a D n/a |
| copper(I) trifluoromethanesulfonate benzene; 2,2'-isopropylidenebis[(4S)-4-tert-butyl-2-oxazoline] In toluene at 0 - 20℃; for 24 - 25h; Product distribution / selectivity; Molecular sieve; | A n/a B n/a C n/a D n/a |

-
-
623-73-4
diazoacetic acid ethyl ester

-
-
3910-55-2
N-(p-methoxybenzylidene)butylamine

-
B
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| methyltrioxorhenium(VII) | A 94% B n/a |

-
-
623-73-4
diazoacetic acid ethyl ester

-
-
53054-00-5
N-hexyl-1-(4-methoxyphenyl)methanimine

-
B
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| methyltrioxorhenium(VII) | A 94% B n/a |


| Conditions | Yield |
|---|---|
| methyltrioxorhenium(VII) | A 93% B n/a |

-
-
623-73-4
diazoacetic acid ethyl ester

-
-
7020-93-1
butyl-(4-methyl-benzyliden)-amine

-
B
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| methyltrioxorhenium(VII) | A 93% B n/a |

-
-
623-73-4
diazoacetic acid ethyl ester

-
-
53053-99-9
N-hexyl-1-(4-methylphenyl)methanimine

-
B
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| methyltrioxorhenium(VII) | A 93% B n/a |

-
-
623-73-4
diazoacetic acid ethyl ester

-
-
836-41-9
p-methoxybenzylidene-phenylamine

-
B
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| methyltrioxorhenium(VII) | A 92% B n/a |

-
-
623-73-4
diazoacetic acid ethyl ester

-
-
1077-18-5
N-benzylidenebutan-1-amine

-
B
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| methyltrioxorhenium(VII) | A 92% B n/a |

-
-
623-73-4
diazoacetic acid ethyl ester

-
-
172367-38-3
N-(4-nitrobenzylidene)hexan-1-amine

-
B
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| methyltrioxorhenium(VII) | A 92% B n/a |


| Conditions | Yield |
|---|---|
| With {(Rh[((1S,1'S,2R,2’R)-1,1’-di-tert-butyl-(2,2’)-diphospholane)]H)3(μ2-H)3(μ3-H)}(BF4)2; hydrogen In methanol at 30℃; under 757.576 Torr; for 1.83333h; Inert atmosphere; Schlenk technique; | 92% |
-
-
623-73-4
diazoacetic acid ethyl ester

-
-
89414-89-1
3α,7β-diacetoxy-24-nor-5β-chol-22-ene

-
A
-
89414-90-4, 89495-32-9, 89495-33-0, 89495-34-1, 91423-34-6
(E)-ethyl 3α,7β-diacetoxy-22,23-methylene-5β-cholan-24-oate

-
B
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| With dirhodium tetraacetate In dichloromethane | A 91.6% B 0.52 g |

-
-
623-73-4
diazoacetic acid ethyl ester

-
-
764-13-6
2,5-Dimethyl-2,4-hexadiene

-
A
-
97-41-6
ethyl 2,2-dimethyl-3-(2-methylpropenyl)cyclopropanecarboxylate

-
B
-
623-91-6
diethyl Fumarate

-
C
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| hexarhodium hexadecacarbonyl at 60℃; for 7h; | A 91% B n/a C n/a |

-
-
623-73-4
diazoacetic acid ethyl ester

-
-
25105-94-6
N-(4-nitrophenylmethylidene)-n-butylamine

-
B
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| methyltrioxorhenium(VII) | A 91% B n/a |

-
-
623-73-4
diazoacetic acid ethyl ester

-
-
131559-05-2
N-tert-butyliminoaniline

-
B
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| copper(II) bis(trifluoromethanesulfonate) In dichloromethane at -25℃; | A 90% B n/a |


| Conditions | Yield |
|---|---|
| With [1-(3-sulfonic acid)]propyl-3-methylimidazolium hydrogen sulfate at 120℃; for 1h; Reagent/catalyst; Microwave irradiation; | 88.39% |
| With sulfuric acid | |
| With sulfuric acid; benzene |
-
-
623-73-4
diazoacetic acid ethyl ester

-
-
110-83-8
cyclohexene

-
A
-
52917-64-3
ethyl 7-norcaranecarboxylate

-
B
-
623-91-6
diethyl Fumarate

-
C
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| hexarhodium hexadecacarbonyl at 25℃; for 7h; | A 88% B n/a C n/a |
| ruthenacarborane 3 at 60℃; for 4h; | A 79 % Chromat. B n/a C n/a |

-
-
292638-84-7
styrene

-
-
623-73-4
diazoacetic acid ethyl ester

-
A
-
946-38-3, 946-39-4, 34702-96-0, 34702-97-1, 34703-00-9, 34716-60-4, 63038-62-0, 63038-63-1, 97-71-2
ethyl 2-phenylcyclopropylcarboxylate

-
B
-
623-91-6
diethyl Fumarate

-
C
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| hexarhodium hexadecacarbonyl at 25℃; for 7h; | A 87% B n/a C n/a |
| ruthenacarborane 3 at 60℃; for 4h; | A 93 % Chromat. B n/a C n/a |
| With palladium(II) trimethylacetate In toluene at 30℃; for 0.65h; stereoselective reaction; | A 83.16 %Chromat. B n/a C n/a |
| With phenol In dichloromethane at 20℃; Inert atmosphere; | A 72 %Spectr. B n/a C n/a |

-
-
623-73-4
diazoacetic acid ethyl ester

-
-
111-34-2
-butyl vinyl ether

-
A
-
78932-45-3
ethyl 2-butoxycyclopropanecarboxylate

-
B
-
623-91-6
diethyl Fumarate

-
C
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| hexarhodium hexadecacarbonyl at 25℃; for 7h; | A 87% B n/a C n/a |
| ruthenacarborane 1 at 60℃; for 4h; | A 93 % Chromat. B n/a C n/a |

-
-
623-73-4
diazoacetic acid ethyl ester

-
-
538-51-2
benzylidene phenylamine

-
-
20414-50-0, 20414-52-2, 49790-76-3
ethyl trans-1,3-diphenylaziridine-2-carboxylate

-
B
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| methyltrioxorhenium(VII) | A 87% B n/a |

-
-
623-73-4
diazoacetic acid ethyl ester

-
-
91-78-1
1,3,5-triphenylhexahydro-1,3,5-triazine

-
A
-
147454-97-5
ethyl 1-phenylaziridine-2-carboxylate

-
B
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| copper(II) bis(trifluoromethanesulfonate) In dichloromethane at 0℃; for 4h; | A 85% B n/a |

-
-
623-73-4
diazoacetic acid ethyl ester

-
-
4784-77-4, 29576-14-5, 39616-19-8
(E/Z)-crotyl bromide

-
A
-
79357-22-5
Ethyl 2-bromo-3-methyl-4-pentenoate

-
B
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| With [Ru(CO)(ttppp)] In dichloromethane at 20℃; Inert atmosphere; optical yield given as %de; | A 84% B n/a |

-
-
110-87-2
3,4-dihydro-2H-pyran

-
-
623-73-4
diazoacetic acid ethyl ester

-
A
-
72229-08-4
ethyl 2-oxabicyclo<4.1.0>heptane-7-carboxylate

-
B
-
623-91-6
diethyl Fumarate

-
C
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| hexarhodium hexadecacarbonyl at 25℃; for 1.5h; | A 82% B n/a C n/a |

-
-
623-73-4
diazoacetic acid ethyl ester

-
-
928-49-4
hex-3-yne

-
A
-
623-91-6
diethyl Fumarate

-
C
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| copper(I) hydrotris(3,5-dimethylpyrazol-1-yl)borate In 1,2-dichloro-ethane at 20℃; | A n/a B 82% C n/a |

-
-
623-73-4
diazoacetic acid ethyl ester

-
-
1458-98-6
2-methyl-3-bromo-1-propene

-
B
-
623-91-6
diethyl Fumarate

-
C
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| With [Ru(CO)(ttppp)] In dichloromethane at 20℃; Inert atmosphere; optical yield given as %de; | A 81% B n/a C n/a |
| With (5,10,15,20-tetramesitylporphyrinato)ruthenium(II) carbonyl In dichloromethane at 20℃; Inert atmosphere; optical yield given as %de; | A 6% B n/a C n/a |


| Conditions | Yield |
|---|---|
| In propan-1-ol at 55℃; for 16h; | 100% |
| In isopropyl alcohol at 55℃; for 18h; | 76% |
| In isopropyl alcohol at 55℃; for 6h; | 60.8% |

| Conditions | Yield |
|---|---|
| With 4-cyanobenzaldehyde In Petroleum ether at 20℃; for 16h; Reagent/catalyst; Sealed tube; Irradiation; | 100% |
| With dibenzoyl peroxide | |
| With dibenzoyl peroxide |

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 1.5h; | 100% |
| In acetonitrile at 20℃; for 1.5h; | 100% |

| Conditions | Yield |
|---|---|
| With diphenyldisulfane In hexane for 24h; Isomerization; Irradiation; reflux; | 99% |
| With C16H25N3O2S In acetonitrile at 80℃; for 16h; | 99% |
| With triphenylphosphine In water at 120℃; for 30h; Product distribution; Mechanism; other phosphines; | 80% |

-
-
141-05-9
Diethyl maleate

-
-
146328-23-6
diethyl N-hydroxyaspartate

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium sulfate In ethanol | 99% |

| Conditions | Yield |
|---|---|
| With potassium acetate In 1,2-dichloro-ethane at 50℃; for 24h; | 99% |
| With C50H40N4O2Ru In dichloromethane at 20℃; for 4h; diastereoselective reaction; | 95% |
| With trifluorormethanesulfonic acid In dichloromethane at 23℃; diastereoselective reaction; | 77% |

-
-
30433-91-1
[2-(2-thyenyl)ethyl]amine

-
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 16h; | 99% |
| In acetonitrile at 20℃; for 16h; | 99% |

-
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| With 2-(diphenylphosphino)-N-((S)-3-methyl-1-oxo-1-(((S)-quinolin-4-yl((1S,2S,4S,5R)-5-vinylquinuclidin-2-yl)methyl)amino)butan-2-yl)benzamide; silver carbonate In toluene at 20℃; Inert atmosphere; enantioselective reaction; | 99% |

-
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| With 2-(diphenylphosphino)-N-((S)-3-methyl-1-oxo-1-(((S)-quinolin-4-yl((1S,2S,4S,5R)-5-vinylquinuclidin-2-yl)methyl)amino)butan-2-yl)benzamide; silver carbonate In toluene at 20℃; for 48h; Inert atmosphere; enantioselective reaction; | 99% |
-
-
66646-88-6
methyl 2-(benzylideneamino)acetate

-
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| With N-((S)-3,3-dimethyl-1-oxo-1-(((S)-1-phenylethyl)amino)butan-2-yl)-2-(diphenylphosphino)benzamide; triethylamine; silver carbonate In toluene at 20℃; for 3h; Reagent/catalyst; Inert atmosphere; enantioselective reaction; | 99% |
| With 2-(diphenylphosphino)-N-((S)-3-methyl-1-oxo-1-(((S)-quinolin-4-yl((1S,2S,4S,5R)-5-vinylquinuclidin-2-yl)methyl)amino)butan-2-yl)benzamide; silver carbonate In toluene at 20℃; for 2h; Reagent/catalyst; Temperature; Inert atmosphere; enantioselective reaction; | 96% |
| With 2-(diphenylphosphino)-N-((S)-2-(((1S,2S)-2-(isobutylamino)-1,2-diphenylethyl)amino)-2-oxo-1-phenylethyl)benzamide; silver carbonate In toluene at 20℃; for 3h; Catalytic behavior; Reagent/catalyst; Solvent; Inert atmosphere; | 94% |
| With N-((S)-2-(((S)-2-(dimethylamino)-1-phenylethyl)amino)-2-oxo-1-phenylethyl)-2-(diphenylphosphino)benzamide; silver carbonate In toluene at 20℃; for 3h; Inert atmosphere; Darkness; enantioselective reaction; | 93% |
| With (S)-2-(diphenylphosphino)-N-(1-((2-(isobutylamino)benzyl)amino)-3,3-dimethyl-1-oxobutan-2-yl)benzamide; silver(l) oxide In toluene at 20℃; for 4h; Catalytic behavior; Reagent/catalyst; Temperature; stereoselective reaction; | 90% |

-
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| With N-((R)-2-(((S)-2-(dimethylamino)-1-phenylethyl)amino)-2-oxo-1-phenylethyl)-2-(diphenylphosphino)benzamide; silver carbonate In toluene at 20℃; for 3h; Inert atmosphere; Darkness; enantioselective reaction; | 99% |
| With 2-(diphenylphosphino)-N-((R)-3-methyl-1-oxo-1-(((R)-quinolin-4-yl((1S,2S,4S,5R)-5-vinylquinuclidin-2-yl)methyl)amino)butan-2-yl)benzamide; silver carbonate In toluene at 20℃; Inert atmosphere; enantioselective reaction; | 91% |
-
-
479499-37-1
(4-fluorobenzylideneamino)acetic acid methyl ester

-
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| With N-((S)-3,3-dimethyl-1-oxo-1-(((S)-1-phenylethyl)amino)butan-2-yl)-2-(diphenylphosphino)benzamide; triethylamine; silver carbonate In toluene at 20℃; for 18h; Reagent/catalyst; Inert atmosphere; enantioselective reaction; | 99% |
| With 2-(diphenylphosphino)-N-((S)-2-(((1S,2S)-2-(isobutylamino)-1,2-diphenylethyl)amino)-2-oxo-1-phenylethyl)benzamide; silver carbonate In toluene at 20℃; for 2h; Inert atmosphere; | 95% |
| With 2-(diphenylphosphino)-N-((S)-3-methyl-1-oxo-1-(((S)-quinolin-4-yl((1S,2S,4S,5R)-5-vinylquinuclidin-2-yl)methyl)amino)butan-2-yl)benzamide; silver carbonate In toluene at 20℃; Inert atmosphere; enantioselective reaction; | 93% |
| With (S)-2-(diphenylphosphino)-N-(1-((2-(isobutylamino)benzyl)amino)-3,3-dimethyl-1-oxobutan-2-yl)benzamide; silver(l) oxide In toluene at 20℃; for 2h; stereoselective reaction; | 90% |
| With N-((S)-2-(((S)-2-(dimethylamino)-1-phenylethyl)amino)-2-oxo-1-phenylethyl)-2-(diphenylphosphino)benzamide; silver carbonate In toluene at 20℃; for 2.5h; Inert atmosphere; Darkness; enantioselective reaction; | 84% |
-
-
479499-37-1
(4-fluorobenzylideneamino)acetic acid methyl ester

-
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| With 2-(diphenylphosphino)-N-((R)-3-methyl-1-oxo-1-(((R)-quinolin-4-yl((1S,2S,4S,5R)-5-vinylquinuclidin-2-yl)methyl)amino)butan-2-yl)benzamide; silver carbonate In toluene at 20℃; Inert atmosphere; enantioselective reaction; | 99% |
| With N-((R)-2-(((S)-2-(dimethylamino)-1-phenylethyl)amino)-2-oxo-1-phenylethyl)-2-(diphenylphosphino)benzamide; silver carbonate In toluene at 20℃; for 3h; Inert atmosphere; Darkness; enantioselective reaction; | 82% |
-
-
76862-09-4
methyl N-(4-chlorobenzylidene)glycinate

-
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| With 2-(diphenylphosphino)-N-((S)-3-methyl-1-oxo-1-(((S)-quinolin-4-yl((1S,2S,4S,5R)-5-vinylquinuclidin-2-yl)methyl)amino)butan-2-yl)benzamide; silver carbonate In toluene at 20℃; Inert atmosphere; enantioselective reaction; | 99% |
| With N-((S)-3,3-dimethyl-1-oxo-1-(((S)-1-phenylethyl)amino)butan-2-yl)-2-(diphenylphosphino)benzamide; triethylamine; silver carbonate In toluene at 20℃; for 5h; Reagent/catalyst; Inert atmosphere; enantioselective reaction; | 99% |
| With 2-(diphenylphosphino)-N-((S)-2-(((1S,2S)-2-(isobutylamino)-1,2-diphenylethyl)amino)-2-oxo-1-phenylethyl)benzamide; silver carbonate In toluene at 20℃; for 2h; Inert atmosphere; | 99% |
| With (S)-2-(diphenylphosphino)-N-(1-((2-(isobutylamino)benzyl)amino)-3,3-dimethyl-1-oxobutan-2-yl)benzamide; silver(l) oxide In toluene at 20℃; for 2h; stereoselective reaction; | 88% |
-
-
76862-09-4
methyl N-(4-chlorobenzylidene)glycinate

-
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| With N-((R)-2-(((S)-2-(dimethylamino)-1-phenylethyl)amino)-2-oxo-1-phenylethyl)-2-(diphenylphosphino)benzamide; silver carbonate In toluene at 20℃; for 2h; Inert atmosphere; Darkness; enantioselective reaction; | 99% |
| With 2-(diphenylphosphino)-N-((R)-3-methyl-1-oxo-1-(((R)-quinolin-4-yl((1S,2S,4S,5R)-5-vinylquinuclidin-2-yl)methyl)amino)butan-2-yl)benzamide; silver carbonate In toluene at 20℃; Inert atmosphere; enantioselective reaction; | 96% |
-
-
126079-18-3
(4-cyanobenzylideneamino)acetic acid methyl ester

-
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| With 2-(diphenylphosphino)-N-((S)-3-methyl-1-oxo-1-(((S)-quinolin-4-yl((1S,2S,4S,5R)-5-vinylquinuclidin-2-yl)methyl)amino)butan-2-yl)benzamide; silver carbonate In toluene at 20℃; Inert atmosphere; enantioselective reaction; | 99% |
| With 2-(diphenylphosphino)-N-((S)-2-(((1S,2S)-2-(isobutylamino)-1,2-diphenylethyl)amino)-2-oxo-1-phenylethyl)benzamide; silver carbonate In toluene at 20℃; for 1h; Inert atmosphere; | 85% |
-
-
40216-71-5
methyl 2-(benzylideneamino)propanoate

-
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| With N-((S)-3,3-dimethyl-1-oxo-1-(((S)-1-phenylethyl)amino)butan-2-yl)-2-(diphenylphosphino)benzamide; triethylamine; silver carbonate In toluene at 20℃; for 18h; Reagent/catalyst; Inert atmosphere; enantioselective reaction; | 99% |
| With 2-(diphenylphosphino)-N-((S)-2-(((1S,2S)-2-(isobutylamino)-1,2-diphenylethyl)amino)-2-oxo-1-phenylethyl)benzamide; silver carbonate In toluene at 20℃; for 40h; Inert atmosphere; | 91% |
| With (S)-2-(diphenylphosphino)-N-(1-((2-(isobutylamino)benzyl)amino)-3,3-dimethyl-1-oxobutan-2-yl)benzamide; silver(l) oxide In toluene at 20℃; for 48h; stereoselective reaction; | 67% |
-
-
68870-85-9
methyl 2-(benzylideneamino)-3-phenylpropionate

-
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| With N-((S)-3,3-dimethyl-1-oxo-1-(((S)-1-phenylethyl)amino)butan-2-yl)-2-(diphenylphosphino)benzamide; triethylamine; silver carbonate In toluene at 20℃; for 24h; Inert atmosphere; enantioselective reaction; | 99% |
| With 2-(diphenylphosphino)-N-((S)-2-(((1S,2S)-2-(isobutylamino)-1,2-diphenylethyl)amino)-2-oxo-1-phenylethyl)benzamide; silver carbonate In toluene at 20℃; for 56h; Inert atmosphere; | 80% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-chlorobenzaldehyde; methoxycarbonylmethylamine With diisopropyl-carbodiimide In toluene for 3h; Inert atmosphere; Stage #2: Diethyl maleate With 2-(diphenylphosphino)-N-((S)-2-(((1S,2S)-2-(isobutylamino)-1,2-diphenylethyl)amino)-2-oxo-1-phenylethyl)benzamide; silver carbonate In toluene at 20℃; for 3h; Inert atmosphere; | 99% |

-
-
756-80-9
O,O-dimethyl phosphorodithioic acid

-
-
141-05-9
Diethyl maleate

-
-
121-75-5
[(dimethoxyphosphinothioyl)thio]-butanedioic acid, diethyl ester

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 10 - 40℃; for 8h; Reagent/catalyst; Green chemistry; | 98% |
| With triethylamine; hydroquinone | |
| With hydroquinone In water at 53℃; for 8h; | |
| at -30 - -25℃; Inert atmosphere; | |
| With triethylamine; hydroquinone |
-
-
114050-31-6
3-Phenyl-propionic acid 2-thioxo-2H-pyridin-1-yl ester

-
-
141-05-9
Diethyl maleate

-
-
146352-28-5
2-Phenethyl-3-(pyridin-2-ylsulfanyl)-succinic acid diethyl ester

| Conditions | Yield |
|---|---|
| In dichloromethane at 0 - 5℃; Irradiation; | 98% |

| Conditions | Yield |
|---|---|
| With acetic acid; zinc for 2h; Ambient temperature; sonication; | 98% |
| With Decaborane; palladium on activated charcoal In methanol at 25℃; for 0.3h; Reduction; | 98% |
| With 1,3-dimethyl-2-phenyl-2,3-dihydro-1H-7-methylbenzo-[d]imidazole; [Zr(2,6-bis(5-methyl-3-phenyl-1H-pyrrol-2-yl)pyridine)2] In benzene-d6 for 8h; UV-irradiation; | 98% |
-
-
29601-98-7
N-benzylhydroxylamine hydrochloride

-
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| Stage #1: formaldehyd; N-benzylhydroxylamine hydrochloride In ethanol; water at 20℃; for 1h; Stage #2: Diethyl maleate In ethanol; water for 3h; Heating / reflux; | 98% |

-
-
756-80-9
O,O-dimethyl phosphorodithioic acid

-
-
141-05-9
Diethyl maleate

-
-
121-75-5
[(dimethoxyphosphinothioyl)thio]-butanedioic acid, diethyl ester

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In dodecane | 98% |


| Conditions | Yield |
|---|---|
| With palladium(II) trimethylacetate; silver pivalate In 1,2-dichloro-ethane at 90℃; sealed tube; optical yield given as %de; stereoselective reaction; | 98% |

-
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| With 2-(diphenylphosphino)-N-((S)-2-(((1S,2S)-2-(isobutylamino)-1,2-diphenylethyl)amino)-2-oxo-1-phenylethyl)benzamide; silver carbonate In toluene at 20℃; for 4.5h; Inert atmosphere; | 98% |
| With 2-(diphenylphosphino)-N-((S)-3-methyl-1-oxo-1-(((S)-quinolin-4-yl((1S,2S,4S,5R)-5-vinylquinuclidin-2-yl)methyl)amino)butan-2-yl)benzamide; silver carbonate In toluene at 20℃; Inert atmosphere; enantioselective reaction; | 95% |
| With N-((S)-2-(((S)-2-(dimethylamino)-1-phenylethyl)amino)-2-oxo-1-phenylethyl)-2-(diphenylphosphino)benzamide; silver carbonate In toluene at 20℃; for 3h; Inert atmosphere; Darkness; enantioselective reaction; | 94% |
| With N-((S)-3,3-dimethyl-1-oxo-1-(((S)-1-phenylethyl)amino)butan-2-yl)-2-(diphenylphosphino)benzamide; triethylamine; silver carbonate In toluene at 20℃; for 6h; Reagent/catalyst; Inert atmosphere; enantioselective reaction; | 85% |
Diethyl maleate Chemical Properties
Structure of Diethyl maleate (CAS NO.141-05-9):
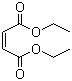
IUPAC Name: diethyl (Z)-but-2-enedioate
Empirical Formula: C8H12O4
Molecular Weight: 172.1785
EINECS: 205-451-9
Index of Refraction: 1.442
Molar Refractivity: 42.71 cm3
Molar Volume: 161.1 cm3
Polarizability: 16.93×10-24cm3
Surface Tension: 33.2 dyne/cm
Density: 1.068 g/cm3
Flash Point: 93.3 °C
Enthalpy of Vaporization: 45.03 kJ/mol
Melting point: -10 ºC
Boiling Point: 214 °C at 760 mmHg
Vapour Pressure: 0.159 mmHg at 25°C
Water Solubility: insoluble
Physical Appearance: colourless liquid
Stability: Stable. Combustible. Incompatible with oxidizing agents, bases, acids, reducing agents.
Product Categories: Fatty Acid Esters (Plasticizer);Functional Materials;Plasticizer
Synonyms of Diethyl maleate (CAS NO.141-05-9): 2-Butenedioic acid (Z)-, diethyl ester ; 4-02-00-02207 (Beilstein Handbook Reference) ; Diethylester kyseliny maleinove ; Ethyl maleate
Canonical SMILES: CCOC(=O)C=CC(=O)OCC
Isomeric SMILES: CCOC(=O)/C=C\C(=O)OCC
InChI: InChI=1S/C8H12O4/c1-3-11-7(9)5-6-8(10)12-4-2/h5-6H,3-4H2,1-2H3/b6-5-
InChIKey: IEPRKVQEAMIZSS-WAYWQWQTSA-N
Diethyl maleate Toxicity Data With Reference
| 1. | skn-rbt 10 mg/24H open MLD | JIHTAB Journal of Industrial Hygiene and Toxicology. 31 (1949),60. | ||
| 2. | skn-rbt 530 mg open MLD | UCDS** Union Carbide Data Sheet. (Industrial Medicine and Toxicology Dept., Union Carbide Corp., 270 Park Ave., New York, NY 10017) 10/29 ,1957. | ||
| 3. | eye-rbt 500 mg open | JIHTAB Journal of Industrial Hygiene and Toxicology. 31 (1949),60. | ||
| 4. | orl-rat LD50:3200 mg/kg | JIHTAB Journal of Industrial Hygiene and Toxicology. 31 (1949),60. | ||
| 5. | ipr-rat LD50:3070 mg/kg | TXAPA9 Toxicology and Applied Pharmacology. 52 (1980),422. | ||
| 6. | skn-rbt LD50:4000 mg/kg | UCDS** Union Carbide Data Sheet. (Industrial Medicine and Toxicology Dept., Union Carbide Corp., 270 Park Ave., New York, NY 10017) 10/29 ,1957. |
Diethyl maleate Consensus Reports
Reported in EPA TSCA Inventory.
Diethyl maleate Safety Profile
Hazard Codes:  Xi
Xi
Risk Statements: 36-43
R36:Irritating to eyes.
R43:May cause sensitization by skin contact.
Safety Statements: 26-36/37-37-24
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37:Wear suitable protective clothing and gloves.
S37:Wear suitable gloves.
S24:Avoid contact with skin.
RIDADR: 3334
WGK Germany: 2
RTECS: ON1225000
HS Code: 29171990
Moderately toxic by ingestion, skin contact, and intraperitoneal routes. A skin and eye irritant. Combustible when exposed to heat or flame; can react with oxidizing materials. To fight fire, use CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.
Diethyl maleate Specification
Diethyl maleate's Handling and Storage
Storage: Keep away from heat, sparks, and flame. Keep away from sources of ignition. Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Handling: Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep container tightly closed. Avoid contact with heat, sparks and flame. Avoid ingestion and inhalation. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames.
Related Products
- Diethyl (2-(cyclohexylamino)vinyl)phosphonate
- Diethyl (2-(triethoxysilyl)ethyl)phosphonate
- Diethyl (2,4,6-trifluorophenyl)malonate
- Diethyl (2-oxopropyl)phosphonate
- Diethyl (2-thienylmethyl)phosphonate
- Diethyl (4-cyanobenzyl)phosphonate
- Diethyl (4-nitrobenzyl)phosphonate
- Diethyl (hydroxymethyl)phosphonate
- Diethyl (methylthiomethyl)phosphonate
- Diethyl (tosyloxy)methylphosphonate
- 14106-16-2
- 141068-81-7
- 141079-19-8
- 141080-73-1
- 141-08-2
- 14108-60-2
- 141091-37-4
- 141091-67-0
- 14109-72-9
- 141103-93-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View