-
Name
Dimethylmorpholine
- EINECS 205-509-3
- CAS No. 141-91-3
- Article Data20
- CAS DataBase
- Density 0.863 g/cm3
- Solubility Soluble in water, ethanol, acetone, benzene
- Melting Point -85 °C(lit.)
- Formula C6H13NO
- Boiling Point 146.599 °C at 760 mmHg
- Molecular Weight 115.175
- Flash Point 48.889 °C
- Transport Information UN 1992 3/PG 3
- Appearance colourless liquid
- Safety 16-26-36/37/39-45
- Risk Codes 10-21-41
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 2,6-Dimethylmorpholine;NSC 60704;2,6-Dimethyl-2,3,5,6-tetrahydro-4H-1,4-oxazine;
- PSA 21.26000
- LogP 0.71200
Synthetic route

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen at 200℃; under 150015 Torr; Reagent/catalyst; Temperature; | 95% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide at 120℃; | |
| With ammonium hydroxide at 120℃; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide Erhitzen des Reaktionsprodukts mit H2SO4 auf 180grad; |

| Conditions | Yield |
|---|---|
| With sulfuric acid at 170℃; |

| Conditions | Yield |
|---|---|
| Reaktion ueber mehrere Stufen; |

| Conditions | Yield |
|---|---|
| at 160 - 170℃; |
-
-
63295-51-2
2,6-dimethyl-N-(2-hydroxypropyl)-morpholine

-
A
-
141-91-3
2,6-dimethyl morpholine

| Conditions | Yield |
|---|---|
| With nitrogen In water; SiO2 |
-
-
63295-51-2
2,6-dimethyl-N-(2-hydroxypropyl)-morpholine

-
-
141-91-3
2,6-dimethyl morpholine

| Conditions | Yield |
|---|---|
| In water |

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen at 180℃; under 150015 Torr; Temperature; Overall yield = 66.3 %; |
-
-
110-91-8
morpholine

-
-
67-56-1
methanol

-
A
-
109-02-4
4-methyl-morpholine

-
B
-
141-91-3
2,6-dimethyl morpholine

| Conditions | Yield |
|---|---|
| With Iridium(II)-coordinated mesoporous (4-[5-(4-aminophenyl)-4'-methyl-[1,1'-biphenyl]-3-yl]aniline)-modified Tröger’s base-functionalized polymer at 110℃; for 24h; Catalytic behavior; Reagent/catalyst; Temperature; | A 89 %Chromat. B n/a |

-
-
141-91-3
2,6-dimethyl morpholine

-
-
1118-02-1
trimethylsilyl isocyanate

-
-
139994-85-7
2,6-dimethyl-morpholine-4-carboxylic acid amide

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 20℃; | 100% |
-
-
141-91-3
2,6-dimethyl morpholine

-
-
1001067-06-6
benzyl 3-nitro-1H-1,2,4-triazole-1-carboxylate

-
-
1001067-13-5
benzyl 2,6-dimethylmorpholine-4-carboxylate

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 0.0833333h; | 100% |
-
-
141-91-3
2,6-dimethyl morpholine

-
-
1001067-08-8
9-fluorenylmethyl 3-nitro-1H-1,2,4-triazole-1-carboxylate

-
-
1001067-15-7
(9H-fluoren-9-yl)methyl 2,6-dimethylmorpholine-4-carboxylate

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 0.0833333h; | 100% |
-
-
141-91-3
2,6-dimethyl morpholine

-
-
1001067-09-9
2-(trimethylsilyl)ethyl 3-nitro-1H-1,2,4-triazole-1-carboxylate

-
-
1001067-16-8
2-(trimethylsilyl)ethyl 2,6-dimethylmorpholine-4-carboxylate

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 0.0833333h; | 100% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile Reflux; | 100% |
| With potassium carbonate In acetonitrile Reflux; |
-
-
141-91-3
2,6-dimethyl morpholine

-
-
98183-26-7
Ethylchlor<4-(3-chlorphenyl)-piperazin-1-ylamido>phosphat

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 80℃; | 99% |
-
-
141-91-3
2,6-dimethyl morpholine

-
-
98156-28-6
Ethylchlor<4-(2-chlorphenyl)-piperazin-1-ylamido>phosphat

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 80℃; | 99% |
-
-
141-91-3
2,6-dimethyl morpholine

-
-
98156-50-4
Ethylchlor<4-(3-trifluormethylphenyl)-piperazin-1-ylamido>phosphat

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 80℃; | 99% |
-
-
141-91-3
2,6-dimethyl morpholine

-
-
191926-73-5
(4-chlorophenyl)(3-chloropropyl)methylpropylsilane

| Conditions | Yield |
|---|---|
| With sodium iodide 90-100 deg C, 2 h, then toluene, reflux, 48 h; | 99% |

| Conditions | Yield |
|---|---|
| In ethanol for 3h; Reflux; | 99% |

-
-
141-91-3
2,6-dimethyl morpholine

-
-
946-80-5
(benzyloxy)benzene

-
-
1774-04-5
4-cyclohexyl-2,6-dimethylmorpholine

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In m-xylene at 90℃; under 7500.75 Torr; for 6h; Autoclave; Green chemistry; | 99% |
-
-
141-91-3
2,6-dimethyl morpholine

-
-
98156-25-3
Ethylchlor<<2-(2,6-dichlorphenoxy)-ethyl>-propylamido>phosphat

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 80℃; | 98% |

| Conditions | Yield |
|---|---|
| With dmap In N,N-dimethyl-formamide 1) 0 deg C, 2-3 h, 2) room temperature, 20-24 h; | 98% |
| With dmap In acetonitrile at 0 - 20℃; for 26h; Schlenk technique; Inert atmosphere; |
-
-
141-91-3
2,6-dimethyl morpholine

-
-
637-59-2
1-Bromo-3-phenylpropane

-
-
415957-91-4
1-N-(2',6'-dimethyl-morpholino)-3-phenyl-propane

| Conditions | Yield |
|---|---|
| Heating; | 98% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 90℃; | 98% |
-
-
141-91-3
2,6-dimethyl morpholine

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 150℃; for 0.5h; Microwave irradiation; | 97.7% |
-
-
141-91-3
2,6-dimethyl morpholine

-
-
830-13-7
cyclododecanone

-
-
1593-77-7, 91269-47-5, 91269-48-6
dodemorph

| Conditions | Yield |
|---|---|
| With hydrogen; ZrO2-containing catalyst at 220 - 240℃; under 37503.8 Torr; | 97% |
-
-
141-91-3
2,6-dimethyl morpholine

-
-
1146300-32-4
7-chloro-1-(2,4-difluorophenyl)-1,4-dihydro-8-methyl-6-nitro-4-oxoquinolone-3-carboxylic acid

-
-
1146300-57-3
1-(2,4-difluorophenyl)-1,4-dihydro-7-(2,6-dimethylmorpholin-4-yl)-8-methyl-6-nitro-4-oxoquinoline-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide Microwave irradiation; | 97% |
-
-
141-91-3
2,6-dimethyl morpholine

-
-
191926-75-7
(4-chlorobutyl)(4-chlorophenyl)(3-chloropropyl)methylsilane

| Conditions | Yield |
|---|---|
| With sodium iodide 90-100 deg C, 2xh, then toluene, reflux, 48 h; | 96% |
-
-
141-91-3
2,6-dimethyl morpholine

-
-
191926-72-4
Bis-(4-chloro-phenyl)-(3-chloro-propyl)-methyl-silane

| Conditions | Yield |
|---|---|
| With sodium iodide 90-100 deg C, 2 h, then reflux, 48 h; | 96% |
-
-
141-91-3
2,6-dimethyl morpholine

-
-
98156-27-5
Ethylchlor<<2-(2,4,6-trichlorphenoxy)-ethyl>-propylamido>phosphat

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 80℃; | 95% |
-
-
141-91-3
2,6-dimethyl morpholine

-
-
131968-14-4
2,3,3,4,5,5,6-Heptafluoro-2,6-bis-trifluoromethyl-morpholine

| Conditions | Yield |
|---|---|
| With fluorine at -100 - 25℃; | 95% |
-
-
141-91-3
2,6-dimethyl morpholine

-
-
945536-26-5
4-methyl-N-2-propen-1-yl-N-[2-[tris(1-methylethyl)silyl]ethynyl]-benzenesulfonamide

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; potassium carbonate In tetrahydrofuran at 80℃; Inert atmosphere; | 95% |
-
-
141-91-3
2,6-dimethyl morpholine

-
-
1001067-07-7
2,2,2-trichloroethyl 3-nitro-1H-1,2,4-triazole-1-carboxylate

-
-
1001067-14-6
2,2,2-trichloroethyl 2,6-dimethylmorpholine-4-carboxylate

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 0.0833333h; | 94% |
-
-
141-91-3
2,6-dimethyl morpholine

-
-
332163-80-1
ethyl 4,5-dimethyl-2-(1H-tetrazol-1-yl)thiophene-3-carboxylate

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 80 - 90℃; for 0.5h; | 94% |
-
-
141-91-3
2,6-dimethyl morpholine

| Conditions | Yield |
|---|---|
| With potassium iodide In tetrahydrofuran; N,N,N,N,N,N-hexamethylphosphoric triamide; water; ethyl acetate | 93% |
-
-
141-91-3
2,6-dimethyl morpholine

-
-
59803-61-1
3-chloro-1,2-dimethyl-5-phenylpyrazolium iodide

| Conditions | Yield |
|---|---|
| In ethanol Heating; | 92% |
-
-
141-91-3
2,6-dimethyl morpholine

-
-
191926-74-6
(4-chlorophenyl)(3-chloropropyl)methyl(prop-2-en-1-yl)silane

| Conditions | Yield |
|---|---|
| With sodium iodide 90-100 deg C, 2 h, then toluene, reflux, 48 h; | 92% |
Dimethylmorpholine Consensus Reports
Dimethylmorpholine Specification
The Dimethylmorpholine, with the CAS registry number 141-91-3, is also known as 2,6-Dimethyl-2,3,5,6-tetrahydro-4H-1,4-oxazine. It belongs to the product categories of Building Blocks; Heterocyclic Building Blocks; Morpholines. Its EINECS number is 205-509-3. This chemical's molecular formula is C6H13NO and molecular weight is 115.17. What's more, its systematic name is 2,6-Dimethylmorpholine. Its classification codes are: (1)Mutation data; (2)Skin / Eye Irritant. This chemical is stable at common pressure and temperature, and it should be sealed and stored. Moreover, it should be protected from strong oxidants and acids. It is used as an intermediate for SBR morpholine and also used in the manufacture of corrosion inhibitors, stabilizer for chlorinated solvents, rubless floor polishes, rubber accelerators, germicides.
Physical properties of Dimethylmorpholine are: (1)ACD/LogP: 0.281; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -2.69; (4)ACD/LogD (pH 7.4): -1.36; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 1.00; (8)ACD/KOC (pH 7.4): 1.00; (9)#H bond acceptors: 2; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 21.26 Å2; (13)Index of Refraction: 1.406; (14)Molar Refractivity: 32.816 cm3; (15)Molar Volume: 133.514 cm3; (16)Polarizability: 13.009×10-24cm3; (17)Surface Tension: 24.24 dyne/cm; (18)Density: 0.863 g/cm3; (19)Flash Point: 48.889 °C; (20)Enthalpy of Vaporization: 38.36 kJ/mol; (21)Boiling Point: 146.599 °C at 760 mmHg; (22)Vapour Pressure: 4.6 mmHg at 25°C.
Preparation: this chemical can be prepared by 2-(2-hydroxyl propyl) amine hydrochloride and concentrated sulfuric acid by heating to dehydration, and then add alkali to separate out the product. The end product is got by distillation.
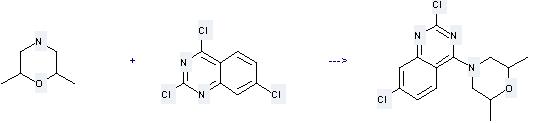
Uses of Dimethylmorpholine: it can be used to produce 2,7-dichloro-4-(2,6-dimethylmorpholino)quinazoline at the temperature of 0 °C. It will need solvent CH2Cl2 with the reaction time of 45 min. The yield is about 74%.
When you are using this chemical, please be cautious about it as the following:
This chemical is flammable, so you should keep it away from sources of ignition - No smoking. It is harmful in contact with skin and has a risk of serious damage to eyes. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need to wear suitable protective clothing and avoid contact with skin and eyes. In case of accident or if you feel unwell, you must seek medical advice immediately (show the label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: O1C(C)CNCC1C
(2)Std. InChI: InChI=1S/C6H13NO/c1-5-3-7-4-6(2)8-5/h5-7H,3-4H2,1-2H3
(3)Std. InChIKey: HNVIQLPOGUDBSU-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rabbit | LD50 | skin | 710uL/kg (.71mL/kg) | SKIN AND APPENDAGES (SKIN): PRIMARY IRRITATION: AFTER TOPICAL EXPOSURE | American Industrial Hygiene Association Journal. Vol. 23, Pg. 95, 1962. |
| rat | LC | inhalation | > 4000ppm/4H (4000ppm) | American Industrial Hygiene Association Journal. Vol. 23, Pg. 95, 1962. | |
| rat | LD50 | oral | 2830mg/kg (2830mg/kg) | Union Carbide Data Sheet. Vol. 11/13/1961. |
Related Products
- Dimethylmorpholine
- 141914-99-0
- 14191-95-8
- 14192-12-2
- 14192-26-8
- 141922-90-9
- 141923-40-2
- 141-92-4
- 141931-13-7
- 14193-38-5
- 141-93-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View