-
Name
Hexadecanal
- EINECS 211-111-0
- CAS No. 629-80-1
- Article Data162
- CAS DataBase
- Density 0.829 g/cm3
- Solubility
- Melting Point 36-38ºC
- Formula C16H32O
- Boiling Point 297.8 °C at 760 mmHg
- Molecular Weight 240.429
- Flash Point 138.9 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Palmitaldehyde(6CI,8CI);1-Hexadecanal;Hexadecanaldehyde;Hexadecylaldehyde;n-Hexadecan-1-al;n-Hexadecanal;
- PSA 17.07000
- LogP 5.66660
Synthetic route
-
-
36653-82-4
1-Hexadecanol

-
-
629-80-1
n-hexadecylaldehyde

| Conditions | Yield |
|---|---|
| With 4-acetylamino-2,2,6,6-tetramethylpiperidine-N-oxyl; toluene-4-sulfonic acid In dichloromethane 1) 0 deg C, 1 h, 2) r.t., 2.5 h; | 100% |
| With 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione In ethyl acetate at 60℃; | 99% |
| With pyridine; 1-chloro-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical In ethyl acetate at 20℃; for 1.5h; | 98% |

| Conditions | Yield |
|---|---|
| With tri-n-butyl-tin hydride; tetrakis(triphenylphosphine) palladium(0) In benzene for 0.166667h; Ambient temperature; | 97% |
| With ammonium hydroxide; formic acid In diethyl ether; chloroform for 0.333333h; Ambient temperature; | 95% |
| With Amberlyst A-26 in the BH4(1-) form in column at 4-5 ml In hexane | 91.04% |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate; dimethyl sulfoxide; sodium iodide at 115℃; for 2.25h; | 96% |
| Multi-step reaction with 3 steps 1: 1.) NaH / 1.) 1,2-dimethoxyethane, 15 min; 2.) dimethoxyethane, 5 h, reflux 2: 74 percent / permaleic acid 3: 1.) trifluoroacetic anhydride; 2.) K2CO3 / 1.) 10 min, r.t.; 2.) 40percent CH3CN/H2O, 10 min, View Scheme |

| Conditions | Yield |
|---|---|
| With ammonium cerium(IV) nitrate; HZSM-5 zeolite In water for 0.166667h; microwave irradiation; | 93% |
| With chromium(VI) oxide; HZSM-5 zeolite for 0.03h; microwave irradiation; | 83% |

| Conditions | Yield |
|---|---|
| With ThxBHO-s-Bu In tetrahydrofuran at 25℃; for 96h; | 87% |
| Stage #1: 1-hexadecylcarboxylic acid With N,O-dimethylhydroxylamine*hydrochloride; N-ethyl-N,N-diisopropylamine; (bis-(2-methoxyethyl)amino)sulfur trufluoride In tetrahydrofuran; dichloromethane at 20℃; for 0.25h; Stage #2: With diisobutylaluminium hydride In tetrahydrofuran; hexane; dichloromethane at -78℃; for 1h; | 83% |
| With Bis(N-methylpiperazinyl) aluminum hydride In tetrahydrofuran for 8h; Heating; | 79% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-octadecanol With oxalyl dichloride; dimethyl sulfoxide In dichloromethane at -10℃; for 0.0833333h; Swern Oxidation; Inert atmosphere; Stage #2: In dichloromethane at -10℃; for 0.25h; Swern Oxidation; Inert atmosphere; | 87% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; diethyl ether; tin(ll) chloride und beim Verseifen des Reaktionsprodukts mit warmem Wasser; |
-
-
25022-78-0
2-hydroxyheptadecanoic acid

-
-
629-80-1
n-hexadecylaldehyde

| Conditions | Yield |
|---|---|
| at 275 - 280℃; |

| Conditions | Yield |
|---|---|
| at 270℃; | |
| at 270℃; Erhitzung; |

| Conditions | Yield |
|---|---|
| With tetrahydrofuran; lithium aluminium tetrahydride |
-
-
5435-44-9, 22243-66-9
(E)-3-Ureido-but-2-enoic acid ethyl ester

-
-
629-80-1
n-hexadecylaldehyde

| Conditions | Yield |
|---|---|
| bei der Destillation; |

| Conditions | Yield |
|---|---|
| With trimethylamine-N-oxide In chloroform | |
| Multi-step reaction with 2 steps 1: 71 percent / AgNO2 / diethyl ether / 0 deg C, 15 h, next room temperature, 8 h 2: 1.) NaH, tert-butyl alcohol, 2.) KMnO4, H3BO3 / 1.) pentane, 20 min, 2.) ethyl acetate, water, 10 min View Scheme |

| Conditions | Yield |
|---|---|
| With lithium dihydrido-bis(2-methoxyethoxo)aluminate In diethyl ether; toluene |
-
-
70583-60-7
1-hexadecanoyl-3,5-dimethyl-1H-pyrazole

-
-
629-80-1
n-hexadecylaldehyde

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In diethyl ether Ambient temperature; |

| Conditions | Yield |
|---|---|
| With borane N,N-diethylaniline complex; dihydrogen peroxide; sodium acetate; benzo[1,3,2]dioxaborole 1.) benzene, 25 deg C, 24 h; Yield given. Multistep reaction; | |
| With borane N,N-diethylaniline complex; dihydrogen peroxide; sodium acetate; benzo[1,3,2]dioxaborole Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With lead(IV) acetate; manganese(II) acetate In benzene Heating; | A 86 % Chromat. B 7 % Chromat. |

| Conditions | Yield |
|---|---|
| With dimethylsulfide borane complex; pyridinium chlorochromate 1) THF, reflux, 1 h, 2.) CH2Cl2, reflux, 1 h; Yield given. Multistep reaction; |
-
-
66271-50-9
1-nitro-hexadecane

-
-
629-80-1
n-hexadecylaldehyde

| Conditions | Yield |
|---|---|
| With potassium permanganate; boric acid; sodium hydride; tert-butyl alcohol 1.) pentane, 20 min, 2.) ethyl acetate, water, 10 min; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With dimethylsulfide borane complex; pyridinium chlorochromate 1) THF, reflux, 1 h, 2.) CH2Cl2, reflux, 1 h; Yield given. Multistep reaction; |
-
-
19366-26-8
1-Phenyl-heptadecan-2-ol

-
-
629-80-1
n-hexadecylaldehyde

| Conditions | Yield |
|---|---|
| With lead(IV) acetate; iodine In benzene Heating; Yield given; | |
| With lead(IV) acetate; iodine In benzene for 6h; Heating; Yield given; |
-
-
120061-38-3
2-hexadecylsulfinyl-3,6-diisopropylpyrazine

-
-
629-80-1
n-hexadecylaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate; trifluoroacetic anhydride 1.) 10 min, r.t.; 2.) 40percent CH3CN/H2O, 10 min,; Yield given. Multistep reaction; |
-
-
629-80-1
n-hexadecylaldehyde

-
-
21204-67-1
methyl (triphenylphosphoranylidene)acetate

-
-
14663-11-7
methyl trans-2-octadecenoate

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 17h; Inert atmosphere; | 100% |
| Wittig reaction; |
-
-
629-80-1
n-hexadecylaldehyde

-
-
86302-43-4
(tert-Butoxycarbonylmethylene)triphenylphosphorane

| Conditions | Yield |
|---|---|
| In dichloromethane at 25℃; for 12h; Wittig Rearrangement; | 99% |
| In dichloromethane at 20℃; for 12h; Wittig Olefination; | 98.8% |
-
-
629-80-1
n-hexadecylaldehyde

-
-
36653-82-4
1-Hexadecanol

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane; nickel boride In diethylene glycol dimethyl ether; N,N-dimethyl-formamide for 1h; Ambient temperature; | 98% |
| With magnesium; tin(ll) chloride In tetrahydrofuran for 0.25h; | 98% |
| With sodium tetrahydroborate; nickel dichloride In tetrahydrofuran at 20℃; for 0.166667h; | 92% |
| With water; nickel dichloride; zinc In N,N-dimethyl-formamide for 5h; Ambient temperature; | 65% |
| With acetic acid; zinc und nachfolgender Verseifung des erhaltenen Cetylacetats mittels alkoholischer Kalilauge; |
-
-
623-73-4
diazoacetic acid ethyl ester

-
-
629-80-1
n-hexadecylaldehyde

-
-
1072879-54-9
3,3',5,5'-tetramethyl-4,4'-dimethoxy benzhydrylamine

-
-
1606993-91-2
ethyl (2S,3S)-1-(bis(4-methoxy-3,5-dimethylphenyl)methyl)-3-pentadecylaziridine-2-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: 3,3',5,5'-tetramethyl-4,4'-dimethoxy benzhydrylamine With triphenylborane In toluene at 80℃; for 0.5h; Inert atmosphere; Sealed tube; Schlenk technique; Stage #2: diazoacetic acid ethyl ester; n-hexadecylaldehyde In toluene Inert atmosphere; Sealed tube; Schlenk technique; Molecular sieve; stereoselective reaction; | 97% |
| Stage #1: 3,3',5,5'-tetramethyl-4,4'-dimethoxy benzhydrylamine With triphenylborane; (R)-t-Bu2VANOL In toluene at 80℃; for 0.5h; Inert atmosphere; Schlenk technique; Stage #2: diazoacetic acid ethyl ester; n-hexadecylaldehyde In toluene at -10℃; for 24h; Molecular sieve; Inert atmosphere; Schlenk technique; enantioselective reaction; | 96.7% |
| Stage #1: 3,3',5,5'-tetramethyl-4,4'-dimethoxy benzhydrylamine With triphenylborane; (2S)-(-)-3,3'-diphenyl-(2,2'-binaphthalene)-1,1'-diol In toluene at 80℃; for 0.5h; Inert atmosphere; Stage #2: diazoacetic acid ethyl ester; n-hexadecylaldehyde In toluene at -10℃; for 24h; Reagent/catalyst; Temperature; Inert atmosphere; Molecular sieve; stereoselective reaction; | 60% |

-
-
420-90-6
3-bromo-3,3-difluropropene

-
-
629-80-1
n-hexadecylaldehyde

-
-
1310740-06-7
3,3-difluorononadec-1-en-4-ol

| Conditions | Yield |
|---|---|
| With indium In tetrahydrofuran; water at 20 - 60℃; Inert atmosphere; | 93% |

| Conditions | Yield |
|---|---|
| With phosphotungstic acid; oxygen at 80℃; for 2h; Green chemistry; chemoselective reaction; | 93% |
-
-
629-80-1
n-hexadecylaldehyde

-
-
103698-50-6
triphenylarsonium salt of bromoacetaldehyde

-
-
51534-37-3
(E)-octadec-2-enal

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran; diethyl ether; water at 25℃; for 17.5h; | 90% |
-
-
629-80-1
n-hexadecylaldehyde

-
-
128899-83-2
(3R,5R)-(+)-2,6-dimethyl-3,5-heptanediol

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene for 3h; Heating; | 90% |

| Conditions | Yield |
|---|---|
| With 3-benzyl-5-(2-hydroxyethyl)-4-methyl-1,3-thiazol-3-ium chloride; triethylamine In ethanol at 80℃; for 3h; Inert atmosphere; | 89% |
| With triethylamine; 4,5-dimethylthiazole bound on chloromethylated polystyrene copolymer In ethanol; chloroform for 96h; Ambient temperature; | 53% |
| With triethylamine In ethanol at 80℃; for 96h; | 51% |
| With 3-benzyl-5-(2-hydroxyethyl)-4-methyl-1,3-thiazol-3-ium chloride In ethanol for 3h; Inert atmosphere; Reflux; |
-
-
629-80-1
n-hexadecylaldehyde

-
-
528867-18-7
(S)-nonadec-1-en-4-ol

| Conditions | Yield |
|---|---|
| In diethyl ether at -78℃; for 4h; | 89% |
Hexadecanal Specification
The Hexadecanal is an organic compound with the formula C16H32O. The IUPAC name of this chemical is hexadecanal. With the CAS registry number 629-80-1, it is also named as Palmitaldehyde.
Physical properties about Hexadecanal are: (1)ACD/LogP: 7.28; (2)# of Rule of 5 Violations: 1; (3)ACD/LogD (pH 5.5): 7.28; (4)ACD/LogD (pH 7.4): 7.28; (5)ACD/BCF (pH 5.5): 201668.2; (6)ACD/BCF (pH 7.4): 201668.2; (7)ACD/KOC (pH 5.5): 218089.84; (8)ACD/KOC (pH 7.4): 218089.84; (9)#H bond acceptors: 1; (10)#Freely Rotating Bonds: 14; (11)Polar Surface Area: 17.07 Å2; (12)Index of Refraction: 1.439; (13)Molar Refractivity: 76.36 cm3; (14)Molar Volume: 289.9 cm3; (15)Polarizability: 30.27×10-24cm3; (16)Surface Tension: 29.8 dyne/cm; (17)Density: 0.829 g/cm3; (18)Flash Point: 138.9 °C; (19)Enthalpy of Vaporization: 53.77 kJ/mol; (20)Boiling Point: 297.8 °C at 760 mmHg; (21)Vapour Pressure: 0.00132 mmHg at 25°C.
Preparation of Hexadecanal: this chemical can be prepared by hexadecanoyl chloride. This reaction will need reagents palladium-barium sulfate, acetone.

Uses of Hexadecanal: it can be used to produce hexadecan-1-ol at temperature of 20 °C. It will need reagents NiCl2, NaBH4 and solvent tetrahydrofuran with reaction time of 10 min. The yield is about 92%.
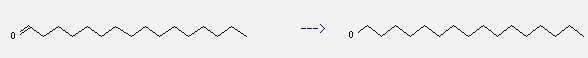
You can still convert the following datas into molecular structure:
(1)SMILES: O=CCCCCCCCCCCCCCCC
(2)InChI: InChI=1/C16H32O/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17/h16H,2-15H2,1H3
(3)InChIKey: NIOYUNMRJMEDGI-UHFFFAOYAM
(4)Std. InChI: InChI=1S/C16H32O/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17/h16H,2-15H2,1H3
(5)Std. InChIKey: NIOYUNMRJMEDGI-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View