-
Name
Indinavir sulfate
- EINECS 207-586-9
- CAS No. 157810-81-6
- Density
- Solubility >100g/L(temperature not stated)
- Melting Point 150-153 °C (dec)
- Formula C36H47N5O4.H2SO4
- Boiling Point 877.9 °C at 760 mmHg
- Molecular Weight 711.87
- Flash Point 484.7 °C
- Transport Information
- Appearance Crystalline solid
- Safety
- Risk Codes
-
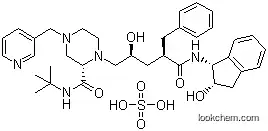
Molecular Structure
- Hazard Symbols
- Synonyms (alphaR,gammaS,2S)-alpha-Benzyl-2-(tert-butylcarbamoyl)-gamma-hydroxy-N-((1S,2R)-2-hydroxy-1-indanyl)-4-(3-pyridylmethyl)-1-piperazinevaleramide sulfate (1:1) (salt);D-erythro-Pentonamide,2,3,5-trideoxy-N-[(1S,- 2R)-2,3-dihydro-2-hydroxy-1H-inden-1- yl]-5-[(2S)-2-[[(1,1-dimethylethyl)amino]- carbonyl]-4-(3-pyridinylmethyl)-1- piperazinyl]-2-(phenylmethyl)-,sulfate (1:1) (salt);Indinavir Sulfate(Anti-AIDS);MK639;
- PSA 201.01000
- LogP 3.95250
Indinavir sulfate History
The Food and Drug Administration (FDA) approved indinavir March 13, 1996, making it the eighth approved antiretroviral. Indinavir was much more powerful than any prior antiretroviral drug; using it with dual NRTIs set the standard for treatment of HIV/AIDS and raised the bar on design and introduction of subsequent antiretroviral drugs. Protease inhibitors changed the very nature of the AIDS epidemic from one of a terminal illness to a somewhat manageable one. Increasingly, it is being replaced by newer drugs that are more convenient to take and less likely to promote resistant virus, such as lopinavir or atazanavir.
Indinavir sulfate Specification
The Indinavir sulfate is an organic compound with the formula C36H47N5O4.H2SO4. The IUPAC name of this chemical is (2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-5-[[(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl]amino]-5-oxopentyl]-N-tert-butyl-4-(pyridin-3-ylmethyl)piperazine-2-carboxamide; sulfuric acid. With the CAS registry number 157810-81-6, it is also named as cannot calculate. The product's categories are Active Pharmaceutical Ingredients; Anti-viral Compounds; Anti-virals; Inhibitors; Intermediates & Fine Chemicals; Non-nucleoside Reverse Transcriptase; Pharmaceuticals; Indinavir. Besides, it is a protease inhibitor used as a component of highly active antiretroviral therapy (HAART) to treat HIV infection and AIDS.
Physical properties about Indinavir sulfate are: (1)ACD/LogP: 2.876; (2)# of Rule of 5 Violations: 1; (3)ACD/LogD (pH 5.5): -1.79; (4)ACD/LogD (pH 7.4): -2.63; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 1; (8)ACD/KOC (pH 7.4): 1; (9)#H bond acceptors: 9; (10)#H bond donors: 4; (11)#Freely Rotating Bonds: 14; (12)Polar Surface Area: 78.45 Å2; (13)Flash Point: 484.7 °C; (14)Enthalpy of Vaporization: 133.67 kJ/mol; (15)Boiling Point: 877.9 °C at 760 mmHg; (16)Vapour Pressure: 5.56E-33 mmHg at 25°C.
You can still convert the following datas into molecular structure:
(1)InChI=1S/C36H47N5O4.H2O4S/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43;1-5(2,3)4/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45);(H2,1,2,3,4)/t28?,29-,31-,32+,33-;/m0./s1;
(2)InChIKey=NUBQKPWHXMGDLP-MWKASFQPSA-N;
(3)SmilesCC(C)(C)NC(=O)[C@@H]1CN(CCN1C[C@H](CC(Cc2ccccc2)C(=O)N[C@H]3c4ccccc4C[C@H]3O)O)Cc5cccnc5.OS(=O)(=O)O;
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LD50 | intraperitoneal | > 640mg/kg (640mg/kg) | GASTROINTESTINAL: NAUSEA OR VOMITING | Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 39, Pg. 2167, 1997. |
| dog | LD50 | oral | > 640mg/kg (640mg/kg) | GASTROINTESTINAL: NAUSEA OR VOMITING | Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 39, Pg. 2167, 1997. |
| mouse | LD50 | intraperitoneal | > 5gm/kg (5000mg/kg) | Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 39, Pg. 2167, 1997. | |
| mouse | LD50 | oral | > 5gm/kg (5000mg/kg) | Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 39, Pg. 2167, 1997. | |
| rat | LD50 | intraperitoneal | > 5gm/kg (5000mg/kg) | Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 39, Pg. 2167, 1997. | |
| rat | LD50 | oral | > 5gm/kg (5000mg/kg) | Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 39, Pg. 2167, 1997. |
Related Products
- Indinavir
- Indinavir sulfate
- 1578-14-9
- 15782-05-5
- 1578-29-6
- 157831-75-9
- 1578-33-2
- 157834-21-4
- 15783-70-7
- 157837-31-5
- 157843-41-9
- 15784-35-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View