-
Name
Ionone
- EINECS 232-396-8
- CAS No. 8013-90-9
- Density 0.944 g/cm3
- Solubility insoluble
- Melting Point
- Formula C13H20O
- Boiling Point 254.8 °C at 760 mmHg
- Molecular Weight 192.33
- Flash Point 121.3 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols R42/43:May cause sensitization by inhalation and skin contact.;
- Synonyms Irisone;beta-lonone;Iraldeine;Lonone (mixed isomers);
- PSA 34.14000
- LogP 7.17230
Ionone Chemical Properties
The Molecular Structure of Ionone (CAS NO.8013-90-9):
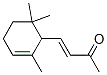
IUPAC Name: (E)-4-(2,6,6-Trimethylcyclohex-2-en-1-yl)but-3-en-2-one
Molecular Formula: C13H20O
Molecular Weight: 192.3 g/mol
Density: 0.944 g/cm3
Boiling Point: 254.8 °C at 760mmHg
Flash Point: 121.3 °C
Molar Volume: 203.6cm3
Molar Refractivity: 61.7cm3
Surface Tension: 33.6dyne/cm
Enthalpy of Vaporization: 49.23kJ/mol
Vapour Pressure: 0.0169mmHg at 25 °C
Index of Refraction: 1.517
XLogP3-AA: 3
H-Bond Acceptor: 1
Rotatable Bond Count: 2
Tautomer Count: 12
Exact Mass: 192.151415
MonoIsotopic Mass: 192.151415
Topological Polar Surface Area: 17.1
Heavy Atom Count: 14
Canonical SMILES: CC1=CCCC(C1C=CC(=O)C)(C)C
Isomeric SMILES: CC1=CCCC(C1/C=C/C(=O)C)(C)C
InChI: InChI=1S/C13H20O/c1-10-6-5-9-13(3,4)12(10)8-7-11(2)14/h6-8,12H,5,9H2,1-4H3/b8-7+
InChIKey: UZFLPKAIBPNNCA-BQYQJAHWSA-N
EINECS: 232-396-8
Product Categories: Biochemistry; Monocyclic Monoterpenes; Terpenes
Ionone Uses
Ionone (CAS NO.8013-90-9) can be used as synthetic food flavouring.
Ionone Production
Ionone can be made from citral and acetone with calcium oxide as a basic heterogeneous catalysis. The nucleophilic addition of the carbanion 3 of acetone 1 to the carbonylgroup on citral 4 is base catalysed. The aldol condensation product 5 eliminates water through the enolate ion 6 to the pseudoionone 7.
.gif)
The reaction proceeds by acid catalysis where the double bond in 7 opens to form the carbocation 8. A rearrangement reaction of the carbocation follows with ring closure to 9. Finally a hydrogen atom can be abstracted from 9 by an acceptor molecule (Y) to form either 10 (extended conjugated system) or 11.
.gif)
Ionone Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | subcutaneous | 2605mg/kg (2605mg/kg) | Behavioral: Anticonvulsant Behavioral: Sleep | Journal of the American Pharmaceutical Association, Scientific Edition. Vol. 46, Pg. 77, 1957. |
| rat | LD50 | oral | 4590mg/kg (4590mg/kg) | Behavioral: Somnolence (General Depressed Activity) Behavioral: Tremor | Food and Cosmetics Toxicology. Vol. 2, Pg. 327, 1964. |
Ionone Consensus Reports
Reported in EPA TSCA Inventory.
Ionone Safety Profile
RTECS: NO0700000
Moderately toxic by subcutaneous route.Mildly toxic by ingestion.Caution: May cause allergic reactions.Mutation data reported.
Ionone Specification
Ionone (CAS NO.8013-90-9) also can be called for Copaiba extract (resinoid) (South American spp. of Copaifera L.) ;
FEMA No. 2595 ; Iraldeine ; Irisone ; Lonone (mixed isomers) ; UNII-8IP66F9ODG ; beta-lonone ; beta-lonone (natural) . When heated to decomposition it emits acrid smoke and fumes.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View