-
Name
Isoprene
- EINECS 201-143-3
- CAS No. 78-79-5
- Article Data431
- CAS DataBase
- Density 0.674 g/cm3
- Solubility Insoluble in water, but soluble in benzene, easily soluble in ethanol, ethyl ether, acetone
- Melting Point 323-329 °C(lit.)
- Formula C5H8
- Boiling Point 34.059 °C at 760 mmHg
- Molecular Weight 68.1185
- Flash Point -40.843 °C
- Transport Information UN 1218 3/PG 1
- Appearance colourless liquid with an aromatic odour
- Safety 53-45-61
- Risk Codes 45-12-52/53-68
-
Molecular Structure
-
Hazard Symbols
 F+,
F+, T
T
- Synonyms Isoprene(8CI);2-Methyl-1,3-butadiene;2-Methylbutadiene;Isopentadiene;NSC 9237;b-Methylbivinyl;1,3-Butadiene,2-methyl-;
- PSA 0.00000
- LogP 1.74850
Synthetic route

| Conditions | Yield |
|---|---|
| With supported heteropolyacid In tert-butyl alcohol at 160℃; under 71257.1 Torr; Pressure; Temperature; | 98.6% |
| With water at 250℃; ueber einen phosphorsaeurehaltigen Katalysator;unter Zusatz von Heptandampf; | |
| With water unter Zusatz von Heptandampf; |

| Conditions | Yield |
|---|---|
| With zeolite H-ZSM-5 In benzene at 90℃; for 10h; | 98% |
| With graphite oxide; zeolite In chloroform-d1 at 150℃; for 0.5h; Sealed tube; |
-
-
91495-68-0
3-benzyloxy-2-methyl-1-butene

-
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| at 750℃; under 0.01 Torr; deprotection; | 96% |
-
-
111-36-4
n-butyl isocyanide

-
-
15450-84-7
1,3-dimethyl-2,5-dihydro-1H-phosphole

-
A
-
1173091-18-3
1,4-dibutyl-2-methyl-1,4,2-diazaphospholidin-3,5-dione

-
B
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| at 20℃; for 16h; | A 94% B n/a |


| Conditions | Yield |
|---|---|
| With piperazine; hydrogen In ethanol at 80℃; under 4500.45 Torr; for 24h; | 92% |
| With ethanol; iron catalyst at 100℃; under 51485.6 Torr; Hydrogenation; | |
| With zinc copper; water at 100℃; under 51485.6 Torr; Hydrogenation; |

| Conditions | Yield |
|---|---|
| With pentacarbonyl(acetonitrile)tungsten In dichloromethane-d2 at 25℃; for 72h; | A 85% B n/a |
| With pentacarbonyl(acetonitrile)tungsten In dichloromethane-d2 for 72h; Ambient temperature; | A 85 % Spectr. B n/a |
-
-
587834-60-4
5-chloro-4-(3-methyl-2-butenyloxy)-2-methylthiopyrimidine

-
A
-
38275-42-2
2-methylthio-5-chloropyrimidine

-
B
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In toluene for 0.8h; Carroll reaction; Electrochemical reaction; | A 85% B n/a |

| Conditions | Yield |
|---|---|
| With pentacarbonyl(acetonitrile)tungsten In dichloromethane-d2 at 25℃; for 24h; | A 84% B n/a |
| With pentacarbonyl(acetonitrile)tungsten In dichloromethane-d2 for 24h; Ambient temperature; | A 84 % Spectr. B n/a |
-
-
753488-56-1
6-O-allyl-1,2-O-isopropylidene-3-O-methallyl-5-O-prenyl-α-D-glucofuranose

-
A
-
78-79-5
isoprene

-
B
-
753488-58-3
6-O-allyl-1,2-O-isopropylidene-3-O-methallyl-α-D-glucofuranose

| Conditions | Yield |
|---|---|
| With (PhSO2)2 In dichloromethane-d2; toluene at 80℃; for 28h; | A n/a B 82% |
-
-
96-17-3, 57456-98-1
2-Methylbutyraldehyde

-
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| With Na-BPO4/ZrP at 350 - 600℃; Temperature; Reagent/catalyst; | 78% |
| With boron(III) phosphate at 51.85℃; Product distribution; Further Variations:; Reagents; Temperatures; Reaction partners; time; Dehydration; |
-
-
867039-46-1
(E)-5-[N-(3-methylbut-2-enyl)-N-(toluene-4-sulfonyl)amino]pent-2-enoic acid methyl ester

-
B
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| iron(III) chloride In dichloromethane at 20℃; for 7h; | A 78% B n/a |

| Conditions | Yield |
|---|---|
| With phosphoric acid; oxalic acid; copper(II) sulfate In water under 9000.9 Torr; Reagent/catalyst; Pressure; Heating; Inert atmosphere; | 74.21% |
-
-
53750-52-0
4-iodo-2-methyl-but-1-ene

-
-
42599-17-7
(E)-1-iodo-1-octene

-
A
-
111-66-0
oct-1-ene

-
C
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| With tert.-butyl lithium In tetrahydrofuran; pentane 1) -78 deg C, 5 h; 2) 23 deg C, 18 h; | A 74% B 8% C 37% |


| Conditions | Yield |
|---|---|
| In water at 300℃; under 760.051 Torr; Catalytic behavior; Reagent/catalyst; Flow reactor; | 73.7% |
| In water at 350℃; under 760.051 Torr; Catalytic behavior; Reagent/catalyst; Pressure; Temperature; Inert atmosphere; | 70% |
| With ammonium molybdate tetrahydrate and phosphoric acid supported on silica In water at 300℃; Reagent/catalyst; | 44.76% |

| Conditions | Yield |
|---|---|
| With 1-fluoro-2-methyl-N,N-diisopropyl-propenylamine In chloroform at 20℃; | A 71% B 16% |

| Conditions | Yield |
|---|---|
| With phosphoric acid; water at 175 - 178℃; under 11401.1 Torr; for 24 - 32h; Product distribution / selectivity; Industry scale; | 67.1% |


| Conditions | Yield |
|---|---|
| at 310℃; under 115 Torr; for 0.133333h; Rate constant; Thermodynamic data; var. of initial pressure, temp., Ea, with addition of propene, toluene; | A n/a B 62.3% |

| Conditions | Yield |
|---|---|
| at 555.9℃; Product distribution; isomerization at various degrees of compression; | A 59% B 17% |
| at 204.6℃; under 21 Torr; for 2h; Kinetics; Product distribution; Thermodynamic data; Ea; further times; further temperatures; |
-
-
50-00-0
formaldehyd

-
-
115-11-7
isobutene

-
A
-
16302-35-5
4-methyl-3,6-dihydro-2H-pyran

-
B
-
2270-61-3
4-methyl-3,4-dihydro-2H-pyran

-
C
-
2568-33-4
3-methyl-butane-1,3-diol

-
D
-
96-39-9
methylcyclopentadiene

-
E
-
219811-94-6
3,4,4-trimethylcyclohexene

-
F
-
590-86-3
isovaleraldehyde

-
G
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| With niobium phosphate at 299.84℃; for 3.33333h; Temperature; | A n/a B n/a C n/a D n/a E n/a F n/a G 57% |

-
-
50904-09-1
2-<(-3-methyl-2-butenyl)oxy>tropone

-
A
-
50904-13-7
3-(1,1-dimethyl-2-propenyl)tropolone

-
B
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| at 140℃; for 2h; pyrolysis; | A 50% B 10 % Chromat. |
| With pyrolysis at 140℃; for 2h; Mechanism; other alkenyloxytropones; | A 50% B 10 % Chromat. |

| Conditions | Yield |
|---|---|
| 100% |
-
-
421-59-0
trifluoromethyldiiodophosphine

-
-
78-79-5
isoprene

-
-
128538-08-9
4-Methyl-1,2-bis-trifluoromethyl-1,2,3,6-tetrahydro-[1,2]diphosphinine

| Conditions | Yield |
|---|---|
| With tin(ll) chloride In tetrahydrofuran | 100% |
-
-
4233-33-4
4-Phenyl-1,2,4-triazolidine-3,5-dione

-
-
78-79-5
isoprene

-
-
10316-49-1
3-methyl-8-phenyl-1,6,8-triazabicyclo[4.3.0]non-3-ene-7,9-dione

| Conditions | Yield |
|---|---|
| In dichloromethane at -10℃; | 100% |

| Conditions | Yield |
|---|---|
| In dichloromethane at -10℃; | 100% |
-
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| C48H58NOP2Si2Y; N,N'-dimethylaniliniumtetrakis(pentafluorophenyl)borate In chlorobenzene at 50℃; for 0.166667h; Product distribution / selectivity; | 100% |
| C48H58NOP2Si2Y; N,N'-dimethylaniliniumtetrakis(pentafluorophenyl)borate In chlorobenzene at 20℃; for 0.333333h; Product distribution / selectivity; | 100% |
| C48H58NOP2Si2Y; N,N'-dimethylaniliniumtetrakis(pentafluorophenyl)borate In chlorobenzene at 20℃; for 1h; Product distribution / selectivity; | 100% |
-
-
292638-84-7
styrene

-
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| With supramolecular complex of potassium with 18-crown-6 In tetrahydrofuran at 10℃; for 0.0833333h; | 100% |
-
-
292638-84-7
styrene

-
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| With supramolecular complex of potassium with 18-crown-6 In tetrahydrofuran at 10℃; for 0.0833333h; | 100% |
-
-
292638-84-7
styrene

-
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| With supramolecular complex of potassium with 18-crown-6 In tetrahydrofuran at 10℃; for 0.0833333h; | 100% |
-
-
292638-84-7
styrene

-
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| With supramolecular complex of potassium with 18-crown-6 In tetrahydrofuran at 10℃; for 0.0833333h; | 100% |
-
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| With gadolinium metallocene-based catalyst; triisobutylaluminum; trityl tetrakis(pentafluorophenyl)borate In toluene at 20℃; for 1h; | 100% |
- poly(methyl methacrylate), with 3,97 3-(bromomethyl)phenyl end groups, Mn 1.24E4 Da by SEC, Mn 1.32E4 by osmometry, Mn 1.36E4 Da by NMR, Mw/Mn 1.03; monomer(s): methyl methacrylate; 1,1-bis[3-(tert-butyldimethylsilyloxymethyl)phenyl]ethylene
-
poly(methyl methacrylate), with 3,97 3-(bromomethyl)phenyl end groups, Mn 1.24E4 Da by SEC, Mn 1.32E4 by osmometry, Mn 1.36E4 Da by NMR, Mw/Mn 1.03; monomer(s): methyl methacrylate; 1,1-bis[3-(tert-butyldimethylsilyloxymethyl)phenyl]ethylene

-
-
78-79-5
isoprene

- poly[(methyl methacrylate)-b-isoprene], asymmetric star-branched, 5-arm, Mn 5.98E4 Da, Mw 5.61E4 Da, Mw/Mn 1.02; monomer(s): methyl methacrylate; 1,1-bis[3-(tert-butyldimethylsilyloxymethyl)phenyl]ethylene; isoprene
-
poly[(methyl methacrylate)-b-isoprene], asymmetric star-branched, 5-arm, Mn 5.98E4 Da, Mw 5.61E4 Da, Mw/Mn 1.02; monomer(s): methyl methacrylate; 1,1-bis[3-(tert-butyldimethylsilyloxymethyl)phenyl]ethylene; isoprene

| Conditions | Yield |
|---|---|
| Stage #1: isoprene Stage #2: poly(methyl methacrylate), with 3,97 3-(bromomethyl)phenyl end groups, Mn 1.24E4 Da by SEC, Mn 1.32E4 by osmometry, Mn 1.36E4 Da by NMR, Mw/Mn 1.03; monomer(s): methyl methacrylate; 1,1-bis[3-(tert-butyldimethylsilyloxymethyl)phenyl]ethylene In tetrahydrofuran at -78℃; for 1h; | 100% |
-
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| With [Me2Si(C5Me4)(μ-PC6H11)Y(CH2SiMe3)]2; trityl tetrakis(pentafluorophenyl)borate In chlorobenzene at 25℃; for 2h; | 100% |
-
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| With (η5-C5Me4(SiMe3))′Y(CH2SiMe3)2(thf); trityl tetrakis(pentafluorophenyl)borate In chlorobenzene at 25℃; for 2h; | 100% |
-
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| With [Me2Si(C5Me4)(μ-PC6H11)Y(CH2SiMe3)]2; trityl tetrakis(pentafluorophenyl)borate In chlorobenzene at 20℃; for 2h; | 100% |
-
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| With (C5Me4nPr)Nd(BH4)2(THF)2; dibutylmagnesium In n-heptane; toluene at 50℃; for 20h; | 100% |
-
-
106-99-0
buta-1,3-diene

-
-
78-79-5
isoprene

- poly(isoprene-block-butadiene), with 33 mol% polyisoprene and 67 mol% polybutadiene, > 99% of Z-1,4-double bonds in polyisoprene and 99% of Z-1,4-double bonds in polybutadiene, Mn 160000, Mw/Mn 1.13 by GPC; monomer(s): 1,3-butadiene; isoprene
-
poly(isoprene-block-butadiene), with 33 mol% polyisoprene and 67 mol% polybutadiene, > 99% of Z-1,4-double bonds in polyisoprene and 99% of Z-1,4-double bonds in polybutadiene, Mn 160000, Mw/Mn 1.13 by GPC; monomer(s): 1,3-butadiene; isoprene

| Conditions | Yield |
|---|---|
| Stage #1: buta-1,3-diene With triisobutylaluminum; trityl tetrakis(pentafluorophenyl)borate; bis(2-diphenylphosphinophenyl)amine-based yttrium complex In toluene at -10 - 25℃; Stage #2: isoprene In toluene at 20℃; for 0.333333h; Further stages.; | 100% |
-
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| With trityl tetrakis(pentafluorophenyl)borate; bis(2-diphenylphosphinophenyl)amine-based yttrium complex In chlorobenzene at 0℃; for 3h; | 100% |
-
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| With trityl tetrakis(pentafluorophenyl)borate; bis(2-diphenylphosphinophenyl)amine-based yttrium complex In chlorobenzene at 80℃; for 0.0833333h; | 100% |
-
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| With trityl tetrakis(pentafluorophenyl)borate; bis(2-diphenylphosphinophenyl)amine-based scandium complex In chlorobenzene at 20℃; for 1h; | 100% |
-
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| With trityl tetrakis(pentafluorophenyl)borate; bis(2-diphenylphosphinophenyl)amine-based yttrium complex In chlorobenzene at 20℃; for 1h; | 100% |
-
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| With trityl tetrakis(pentafluorophenyl)borate; bis(2-diphenylphosphinophenyl)amine-based lutetium complex In chlorobenzene at 20℃; for 1h; | 100% |
-
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| With trityl tetrakis(pentafluorophenyl)borate; bis(2-diphenylphosphinophenyl)amine-based yttrium complex In chlorobenzene at 20℃; for 0.333333h; | 100% |
-
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| diethylaluminium chloride In hexane at 40℃; for 24h; | 100% |
-
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| diethylaluminium chloride In hexane at 40℃; for 24h; | 100% |
-
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| diethylaluminium chloride In hexane at 40℃; for 24h; | 100% |
-
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| diethylaluminium chloride In hexane at 40℃; for 24h; | 100% |
-
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| diethylaluminium chloride In hexane at 40℃; for 24h; | 100% |
-
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| diethylaluminium chloride In hexane at 40℃; for 24h; | 100% |
-
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| diethylaluminium chloride In hexane at 40℃; for 24h; | 100% |
-
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| diethylaluminium chloride In hexane at 40℃; for 24h; | 100% |
Isoprene Specification
Isoprene is an organic compound with the formula C5H8, and its systematic name is the same with the product name. With the CAS registry number 78-79-5, it is also named as 2-Methyl-1,3-butadiene. Its EINECS number is 201-143-3. In addition, the molecular weight is 68.12. Its classification codes are: (1)Mutation data; (2)Tumor data. This product should be sealed and stored in a cool place. Moreover, it should be protected from light, heat and fire. It is highly volatile because of its low boiling point. It is the most abundant hydrocarbon measurable in the breath of humans. This compound was first isolated by thermal decomposition of natural rubber. It is most readily available industrially as a byproduct of the thermal cracking of naphtha or oil, as a side product in the production of ethylene. This chemical is used in manufacture of ''synthetic'' rubber, butyl rubber; copolymer in production of elastomers.
Physical properties of Isoprene are: (1)ACD/LogP: 2.347; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.35; (4)ACD/LogD (pH 7.4): 2.35; (5)ACD/BCF (pH 5.5): 35.78; (6)ACD/BCF (pH 7.4): 35.78; (7)ACD/KOC (pH 5.5): 450.51; (8)ACD/KOC (pH 7.4): 450.51; (9)#H bond acceptors: 0; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 1; (12)Index of Refraction: 1.4; (13)Molar Refractivity: 24.507 cm3; (14)Molar Volume: 101.058 cm3; (15)Polarizability: 9.715×10-24cm3; (16)Surface Tension: 17.02 dyne/cm; (17)Density: 0.674 g/cm3; (18)Flash Point: -40.843 °C; (19)Enthalpy of Vaporization: 26.765 kJ/mol; (20)Boiling Point: 34.059 °C at 760 mmHg; (21)Vapour Pressure: 548.96 mmHg at 25°C.
Preparation: this chemical can be prepared by 2-methyl-but-3-en-2-ol at the temperature of 20 °C. This reaction will need reagent N,N-diisopropyl-1-fluoro-2-methylpropenamine and solvent CHCl3. The yield is about 71%.
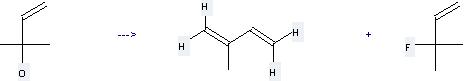
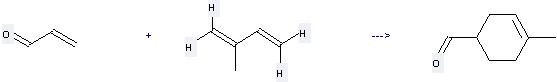
Uses of Isoprene: it can be used to produce 4-methyl-cyclohex-3-enecarbaldehyde at the ambient temperature. It will need reagent methylrhenium trioxide and solvent H2O with the reaction time of 2.5 hours. The yield is about 91%.
When you are using this chemical, please be cautious about it as the following:
This chemical is extremely flammable. It may cause cancer and has a possible risk of irreversible effects. This compound is harmful to aquatic organisms as it may cause long-term adverse effects in the aquatic environment. In case of accident or if you feel unwell, you need to seek medical advice immediately (show the label where possible). It should be avoided exposure, and you need to obtain special instructions before use. You must avoid releasing it to the environment, and you need to refer to special instructions/safety data sheet.
You can still convert the following datas into molecular structure:
(1)SMILES: CC(=C)C=C
(2)Std. InChI: InChI=1S/C5H8/c1-4-5(2)3/h4H,1-2H2,3H3
(3)Std. InChIKey: RRHGJUQNOFWUDK-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LC50 | inhalation | 139gm/m3/2H (139000mg/m3) | LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 9(1), Pg. 36, 1965. |
| rat | LC50 | inhalation | 180gm/m3/4H (180000mg/m3) | BEHAVIORAL: GENERAL ANESTHETIC LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Russian Pharmacology and Toxicology Vol. 31, Pg. 162, 1968. |
Related Products
- Isoprene
- Isoprene dibromide
- 78800-52-9
- 78806-60-7
- 78806-74-3
- 78807-78-0
- 78-80-8
- 78811-27-5
- 788116-25-6
- 78812-64-3
- 78813-13-5
- 788136-89-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View