-
Name
Levofloxacin hydrochloride
- EINECS 600-146-0
- CAS No. 100986-85-4
- Article Data41
- CAS DataBase
- Density 1.48 g/cm3
- Solubility Slightly soluble in water or methanol. Soluble in glacial acetic acid or dichloromethane
- Melting Point 218 °C
- Formula C18H20FN3O4
- Boiling Point 571.495 °C at 760 mmHg
- Molecular Weight 361.373
- Flash Point 299.43 °C
- Transport Information
- Appearance Slight yellow powder
- Safety 26-36/37/39-36
- Risk Codes 22-42/43-68-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Elequine;(-)-Ofloxacin;7H-Pyrido[1,2,3-de]-1,4-benzoxazine-6- carboxylic acid,9-fluoro-2,3-dihydro-3- methyl-10-(4-methyl-1-piperazinyl)-7-oxo-,(3S)-;Levaquin (TN);Levofloxacino [INN-Spanish];Levofloxacin (JAN/USAN);Levaquin;Levofloxacin [USAN:INN:JAN];Levofloxacine [INN-French];(S)-Ofloxacin;Ofloxacin S-(-)-form;Iquix;(S)-9-Fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido(1,2,3-de)-1,4-benzoxazine-6-carboxylic acid;Levofloxacinum [INN-Latin];7H-Pyrido(1,2,3-de)-1,4-benzoxazine-6-carboxylic acid, 2,3-dihydro-9-fluoro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-, (S)-;(S)-(-)-Ofloxacin;8-fluoro-3-methyl-9-(4-methyl-piperazin-1-yl)-6-oxo-2,3-dihydro-6H-1-oxa-3a-aza-phenalene-5-carboxylic acid;Levoflaoxacin;Levofloxacincas;
- PSA 75.01000
- LogP 1.54690
Synthetic route

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water at 55℃; for 24h; Reagent/catalyst; Time; Temperature; Solvent; | 97.1% |
| With nano iron oxide on ZrO2 coated sulfonic acid In water for 0.366667h; Reflux; | 96% |
| at 150℃; Microwave irradiation; | 89% |
-
-
50398-09-9, 51545-09-6, 34352-59-5
N-methylpiperazine dihydrochloride

-
-
100986-89-8
levofloxacin Q-acid

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| Stage #1: N-methylpiperazine dihydrochloride; levofloxacin Q-acid With boron trifluoride-tetrahydrofuran complex; triethylamine In acetonitrile at 20℃; for 24.5h; Stage #2: With methanol for 24h; Reflux; Stage #3: In ethanol at 20℃; for 16h; | 92% |
-
-
109-01-3
1-methyl-piperazine

-
-
106939-34-8
ethyl (S)-(-)-9,10-Difluoro-3-Methyl-7-Oxo-2,3-Dihydro-7H-Pyrido[1,2,3-de]-[1,4]Benzoxazine-6-Carboxylate

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| With acetic acid In water; N,N-dimethyl-formamide at 70 - 105℃; for 10h; Temperature; Solvent; Reagent/catalyst; | 85.7% |
-
-
177472-29-6
(S)-ethyl 6,8-difluoro-1-(1-hydroxypropan-2-yl)-7-(4-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylate

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| Stage #1: (S)-ethyl 6,8-difluoro-1-(1-hydroxypropan-2-yl)-7-(4-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylate With potassium hydroxide; ethanol; water at 20℃; for 2.5h; Heating / reflux; Stage #2: With acetic acid In ethanol at 0 - 20℃; for 2h; pH=7; Product distribution / selectivity; | 78% |

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol; water at 83℃; for 36h; | 77.9% |
| With hydrogenchloride In ethanol; water at 83℃; for 36h; | 75.3% |
-
-
1036016-10-0
(S)-6,8-difluoro-1-(1-hydroxypropan-2-yl)-7-(4-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| Stage #1: (S)-6,8-difluoro-1-(1-hydroxypropan-2-yl)-7-(4-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid With potassium hydroxide In ethanol; water for 3h; Heating / reflux; Stage #2: With acetic acid In ethanol; water at 0 - 20℃; Product distribution / selectivity; | 76% |

-
-
100986-89-8
levofloxacin Q-acid

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| In pyridine; diethyl ether | 74.3% |
-
-
50-00-0
formaldehyd

-
-
117707-40-1
S-(-)-9-fluoro-2,3-dihydro-3-methyl-10-(1-piperazinyl)-7-oxo-7H-pyrido-<1,2,3-de><1,4>benzoxazine-6-carboxylic acid

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| With formic acid In water at 90℃; for 10h; Temperature; | 74.3% |
-
-
109-01-3
1-methyl-piperazine

-
-
129306-33-8
(-)-9,10-difluoro-2,3-dihydro-3-methyl-7-oxo-7H-pyrido<1,2,3-de><1,4>benzoxazine-6-carboxylic acid BF2-chelate

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide for 17h; Ambient temperature; | 52% |

| Conditions | Yield |
|---|---|
| With Bis(2-ethylhexyl)phosphoric acid; sodium dodecyl-sulfate; O,O'-dibenzoyl-L-tartaric acid In octanol; water at 20℃; for 4h; pH=7; Reflux; Resolution of racemate; optical yield given as %ee; enantioselective reaction; | A 8.54% B 34.23% |
| With (2-hydroxypropyl)-α-cyclodextrin In phosphate buffer pH=2.3; capillary electrophoresis; | |
| With Bis(2-ethylhexyl)phosphoric acid; Di-p-toluoyl-L-tartaric acid; O,O'-dibenzoyl-L-tartaric acid In octanol; water at 25℃; for 0.5h; pH=6.86; Resolution of racemate; aq. phosphate buffer; enantioselective reaction; |
-
-
94695-50-8
ethyl 3-(2,3,4,5-tetrafkuorophenyl)-3-oxopropanoate

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: acetic anhydride / 2 h / 110 °C 2: CH2Cl2 / 0.5 h / Ambient temperature 3: 59 percent / 50percent NaH / dimethylsulfoxide / 3 h / Ambient temperature 4: 70 percent / 10percent aq. KOH / tetrahydrofuran / 2 h / 65 - 70 °C 5: 83 percent / pyridine / 12 h / 120 °C View Scheme |
-
-
103995-33-1
3-(2,3,4,5-tetrafluorophenyl)-3-oxo-2-(ethoxymethylene)-propanoic acid ethyl ester

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: CH2Cl2 / 0.5 h / Ambient temperature 2: 59 percent / 50percent NaH / dimethylsulfoxide / 3 h / Ambient temperature 3: 70 percent / 10percent aq. KOH / tetrahydrofuran / 2 h / 65 - 70 °C 4: 83 percent / pyridine / 12 h / 120 °C View Scheme |
-
-
110548-02-2
(+)-ethyl 2-<<<(S)-1-hydroxyprop-2-yl>amino>methylene>-3-oxo-3-(2,3,4,5-tetrafluorophenyl)propionate

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 59 percent / 50percent NaH / dimethylsulfoxide / 3 h / Ambient temperature 2: 70 percent / 10percent aq. KOH / tetrahydrofuran / 2 h / 65 - 70 °C 3: 83 percent / pyridine / 12 h / 120 °C View Scheme |
-
-
110548-03-3
(-)-ethyl 1,4-dihydro-1-<1(S)-(hydroxymethyl)ethyl>-4-oxo-6,7,8-trifluoroquinoline-3-carboxylate

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 70 percent / 10percent aq. KOH / tetrahydrofuran / 2 h / 65 - 70 °C 2: 83 percent / pyridine / 12 h / 120 °C View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: 1-methyl-piperazine; (3S)-9,10-difluoro-3-methyl-7-oxo-2,3-dihydro-7H-pyrido[1,2,3-de][1,4]benzoxazine-6-carboxylic acid-boron difluoride chelate complex With triethylamine In dimethyl sulfoxide at 20℃; for 17h; Stage #2: With triethylamine In ethanol for 8h; Heating / reflux; Stage #3: With hydrogenchloride In water |

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| Stage #1: (-) potassium N-(1-hydroxy-propy-2(S)-yl)-6-fluoro-7-(N-methylpiperazinyl)-8-nitro-quinol-4-one-3-carboxylate With potassium hydroxide In methanol for 2.5h; Heating / reflux; Stage #2: With acetic acid In methanol |

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide | 5.63 g (87.6%) |

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide | 11.8 g (92.4%) |
-
-
109-01-3
1-methyl-piperazine

-
-
107-98-2
1-methoxy-2-propanol

-
-
142-82-5
n-heptane

-
-
100986-89-8
levofloxacin Q-acid

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| 2.98 g (77.3%) |

-
-
100986-89-8
levofloxacin Q-acid

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| In 1-methyl-piperazine; n-heptane |
-
-
109-01-3
1-methyl-piperazine

-
-
109-63-7
trifluoroborane diethyl ether

-
-
100986-89-8
levofloxacin Q-acid

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| With triethylamine In hydrogenchloride; methanol; diethyl ether; dimethyl sulfoxide |


| Conditions | Yield |
|---|---|
| In ethanol; dimethyl sulfoxide |


| Conditions | Yield |
|---|---|
| pH=7; Reagent/catalyst; Resolution of racemate; |

-
-
106939-34-8
ethyl (S)-(-)-9,10-Difluoro-3-Methyl-7-Oxo-2,3-Dihydro-7H-Pyrido[1,2,3-de]-[1,4]Benzoxazine-6-Carboxylate

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: acetic acid; sulfuric acid / water / 4 h / Reflux 2: triethylamine / dimethyl sulfoxide / 8 h / 90 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sulfuric acid; acetic acid / N,N-dimethyl-formamide / 1 h / Reflux; Green chemistry 2: water / 10.5 h / 110 °C / Green chemistry View Scheme | |
| Multi-step reaction with 2 steps 1: sulfuric acid; acetic acid / water / 3 h / 20 °C / Reflux 2: water / 15.5 h / 120 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sulfuric acid; acetic acid / N,N-dimethyl-formamide / 1 h / Reflux 2: water / 10.5 h / 110 °C View Scheme |

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: ammonia / toluene / 4 h / 50 °C 2: toluene / 50 - 100 °C / pH < 7 3: hydrogenchloride / toluene; water / pH < 7 4: potassium fluoride / N,N-dimethyl-formamide / Reflux 5: acetic acid; sulfuric acid / water / 4 h / Reflux 6: triethylamine / dimethyl sulfoxide / 8 h / 90 °C View Scheme |

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: toluene / 50 - 100 °C / pH < 7 2: hydrogenchloride / toluene; water / pH < 7 3: potassium fluoride / N,N-dimethyl-formamide / Reflux 4: acetic acid; sulfuric acid / water / 4 h / Reflux 5: triethylamine / dimethyl sulfoxide / 8 h / 90 °C View Scheme |

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: hydrogenchloride / toluene; water / pH < 7 2: potassium fluoride / N,N-dimethyl-formamide / Reflux 3: acetic acid; sulfuric acid / water / 4 h / Reflux 4: triethylamine / dimethyl sulfoxide / 8 h / 90 °C View Scheme |

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: potassium fluoride / N,N-dimethyl-formamide / Reflux 2: acetic acid; sulfuric acid / water / 4 h / Reflux 3: triethylamine / dimethyl sulfoxide / 8 h / 90 °C View Scheme | |
| Multi-step reaction with 3 steps 1: potassium carbonate / N,N-dimethyl-formamide / 4 h / 100 °C / Green chemistry 2: sulfuric acid; acetic acid / N,N-dimethyl-formamide / 1 h / Reflux; Green chemistry 3: water / 10.5 h / 110 °C / Green chemistry View Scheme | |
| Multi-step reaction with 3 steps 1: potassium carbonate / N,N-dimethyl-formamide / 8 h / 150 °C 2: sulfuric acid; acetic acid / water / 3 h / 20 °C / Reflux 3: water / 15.5 h / 120 °C View Scheme | |
| Multi-step reaction with 3 steps 1: potassium carbonate / N,N-dimethyl-formamide / 4 h / 100 °C 2: sulfuric acid; acetic acid / N,N-dimethyl-formamide / 1 h / Reflux 3: water / 10.5 h / 110 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 24 h / 120 °C 2: potassium carbonate / 16 h / 140 °C 3: hydrogenchloride / ethanol; water / 36 h / 83 °C View Scheme |

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| With GLUTATHIONE In methanol at 23℃; pH=6.5; Kinetics; |

| Conditions | Yield |
|---|---|
| In ethanol at 80℃; for 1h; Temperature; | 94.3% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol; acetone at 20℃; for 24h; | 94% |

-
-
100986-85-4
levofloxacin

-
-
2450-71-7
Propargylamine

| Conditions | Yield |
|---|---|
| Stage #1: levofloxacin With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 20℃; Inert atmosphere; Stage #2: Propargylamine With potassium carbonate In tetrahydrofuran; N,N-dimethyl-formamide at 20℃; for 1h; Inert atmosphere; | 90.7% |
-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| In water; ethyl acetate for 1h; Purification / work up; Heating / reflux; | 90% |
| In 2,8-dimethylnonan-5-one; water for 1h; Purification / work up; Heating / reflux; | 89.6% |
| In acetic acid methyl ester; water for 1h; Purification / work up; Heating / reflux; | 85.6% |
-
-
1202-34-2
di(pyridin-2-yl)amine

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| Stage #1: copper(II) choride dihydrate; levofloxacin With potassium hydroxide In methanol at 20℃; for 0.5h; Stage #2: di(pyridin-2-yl)amine In methanol at 60℃; for 2h; | 83% |

-
-
57-67-0
4-amino-N-(diaminomethylene) benzenesulfonamide

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| With acetic acid In methanol for 2h; Microwave irradiation; Reflux; | 82.94% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol; acetone at 20℃; for 24h; | 81.4% |
-
-
77878-01-4
octadecanoic acid chloromethyl ester

-
-
100986-85-4
levofloxacin

-
A
-
121819-05-4
C37H72O4

-
B
-
1261134-66-0
(3S)-6-[(octadecanoyloxy)methyl] 9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: levofloxacin With potassium carbonate In N,N-dimethyl-formamide at 90℃; for 0.333333h; Stage #2: octadecanoic acid chloromethyl ester In N,N-dimethyl-formamide at 90℃; for 1h; | A n/a B 81% |
| Stage #1: levofloxacin With potassium carbonate In acetonitrile for 8h; Reflux; Stage #2: octadecanoic acid chloromethyl ester With tetra-(n-butyl)ammonium iodide In acetonitrile for 168h; Reflux; |

-
-
54-85-3
isoniazid

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| With trichlorophosphate Reflux; | 81% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 24h; | 80% |
-
-
63-74-1
sulfanilamide

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| With acetic acid In methanol for 2h; Microwave irradiation; Reflux; | 80% |
-
-
61413-67-0
chloromethyl n-dodecanoate

-
-
100986-85-4
levofloxacin

-
A
-
1261134-88-6
C25H48O4

-
B
-
1261134-60-4
(3S)-6-[(dodecanoyloxy)methyl] 9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: levofloxacin With potassium carbonate In N,N-dimethyl-formamide at 90℃; for 0.333333h; Stage #2: chloromethyl n-dodecanoate In N,N-dimethyl-formamide at 90℃; for 1h; | A n/a B 79% |
| Stage #1: levofloxacin With potassium carbonate In acetonitrile for 8h; Reflux; Stage #2: chloromethyl n-dodecanoate With tetra-(n-butyl)ammonium iodide In acetonitrile for 168h; Reflux; |

-
-
100986-85-4
levofloxacin

-
-
61413-69-2
chloromethyl n-hexadecanoate

-
A
-
91360-29-1
C33H64O4

-
B
-
1261134-64-8
(3S)-6-[(hexadecanoyloxy)methyl] 9-fluoro-3,7-dihydro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-2H-[1,4]oxazino-[2,3,4-ij]quinoline-6-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: levofloxacin With potassium carbonate In N,N-dimethyl-formamide at 90℃; for 0.333333h; Stage #2: chloromethyl n-hexadecanoate In N,N-dimethyl-formamide at 90℃; for 1h; | A n/a B 79% |
| Stage #1: levofloxacin With potassium carbonate In acetonitrile for 8h; Reflux; Stage #2: chloromethyl n-hexadecanoate With tetra-(n-butyl)ammonium iodide In acetonitrile for 168h; Reflux; |

-
-
100986-85-4
levofloxacin

-
-
67317-62-8
decanoyloxymethyl chloride

-
A
-
1261134-58-0
(3S)-6-[(decanoyloxy)methyl] 9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylate

-
B
-
76068-80-9
Methylendidecanoat

| Conditions | Yield |
|---|---|
| Stage #1: levofloxacin With potassium carbonate In N,N-dimethyl-formamide at 90℃; for 0.333333h; Stage #2: decanoyloxymethyl chloride In N,N-dimethyl-formamide at 90℃; for 1h; | A 79% B n/a |

-
-
95-14-7
1,2,3-Benzotriazole

-
-
100986-85-4
levofloxacin

-
-
1381763-83-2
(S)-6-(1H-benzo[d][1,2,3]triazole-1-carbonyl)-9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-2H-[1,4]oxazino[2,3,4-ij]quinolin-7(3H)-one

| Conditions | Yield |
|---|---|
| With thionyl chloride In dichloromethane at 20℃; | 79% |

| Conditions | Yield |
|---|---|
| Stage #1: levofloxacin With sodium hydrogencarbonate In water at 25℃; for 0.5h; Stage #2: dimethyltin dichloride In toluene Reflux; | 79% |

| Conditions | Yield |
|---|---|
| Stage #1: copper(II) choride dihydrate; levofloxacin With potassium hydroxide In methanol at 20℃; for 0.5h; Stage #2: bathophenanthroline In methanol at 60℃; for 2h; | 79% |

-
-
127-79-7
sulfamerazina

-
-
100986-85-4
levofloxacin

| Conditions | Yield |
|---|---|
| With acetic acid In methanol for 2h; Microwave irradiation; Reflux; | 78.91% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol; acetone at 20℃; for 24h; | 78.6% |
Levofloxacin History
Levofloxacin is a fluoroquinolone antibiotic, marketed by sanofi aventis under the tradename "TAVANIC". Levaquin is also marketed worldwide for oral and IV use, as well as used in ophthalmic solutions. Daiichi Sankyo had granted an exclusive license to Sanofi-Aventis to make, use and sell pharmaceutical preparations containing levofloxacin in the UK and Mexico under the trade name TAVANIC. Other manufacturers include Novell Pharmaceutical Laboratories (Levores).
Levofloxacin Specification
1. Introduction of Levofloxacin
Levofloxacin is slight yellow powder. The IUPAC Name of this chemical is (S)-7-fluoro-6-(4-methylpiperazin-1-yl)-10-oxo-4-thia-1-azatricyclo[7.3.1.05,13] trideca-5(13),6,8,11-tetraene-11-carboxylic acid. Besides, Levofloxacin belongs to Pharmaceutical material and intermeidates;Active Pharmaceutical Ingredients;Anti-Infective;Antibiotics for Research and Experimental Use;Biochemistry;Quinolones (Antibiotics for Research and Experimental Use);Peptide Synthesis/Antibiotics;Pharmaceutical intermediate.
The Classification Code of it is Anti-Bacterial Agents; Anti-Infective Agents; Anti-infective agents, urinary; Drug / Therapeutic Agent; Enzyme Inhibitors; Nucleic Acid Synthesis Inhibitors; Renal Agents; Reproductive Effect. Levofloxacin is considered soluble to freely soluble in this pH range, as defined by USP nomenclature.
2. Properties of Levofloxacin
Physical properties about Levofloxacin are:
(1)Melting Point: 218 °C ; (2)storage temp.: Store at 0-5 °C ; (3)Index of Refraction: 1.669; (4)Molar Refractivity: 91.09 cm3; (5)Molar Volume: 243.9 cm3; (6)Surface Tension: 70.3 dyne/cm ; (7)Density: 1.48 g/cm3; (8)Flash Point: 299.4 °C; (9)Enthalpy of Vaporization: 90.15 kJ/mol; (10)Boiling Point: 571.5 °C at 760 mmHg; (11)Vapour Pressure of Levofloxacin: 6.7E-14 mmHg at 25 °C.
3. Structure Descriptors of Levofloxacin
(1)SMILES: Fc4cc1c2N(/C=C(\C1=O)C(=O)O)C(COc2c4N3CCN(C)CC3)C
(2)InChI: InChI=1S/C18H20FN3O4/c1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21/h7-8,10H,3-6,9H2,1-2H3,(H,24,25)/t10-/m0/s1
(3)InChIKey: GSDSWSVVBLHKDQ-JTQLQIEISA-N
(4)Canonical SMILES: CC1COC2=C3N1C=C(C(=O)C3=CC(=C2N4CCN(CC4)C)F)C(=O)O
(5)Isomeric SMILES: C[C@H]1COC2=C3N1C=C(C(=O)C3=CC(=C2N4CCN(CC4)C)F)C(=O)O
4. Toxicity of Levofloxacin
Organism Test Type Route Reported Dose (Normalized Dose) Effect Source monkey LD50 oral > 250mg/kg (250mg/kg) SENSE ORGANS AND SPECIAL SENSES: PTOSIS: EYE
BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY)
LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSIONArzneimittel-Forschung. Drug Research. Vol. 42, Pg. 365, 1992. mouse LD50 oral 1803mg/kg (1803mg/kg) SENSE ORGANS AND SPECIAL SENSES: PTOSIS: EYE
BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY)
LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSIONArzneimittel-Forschung. Drug Research. Vol. 42, Pg. 365, 1992. rat LD50 oral 1478mg/kg (1478mg/kg) SENSE ORGANS AND SPECIAL SENSES: PTOSIS: EYE
BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY)
LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSIONArzneimittel-Forschung. Drug Research. Vol. 42, Pg. 365, 1992. women TDLo oral 122mg/kg/10D- (122mg/kg) MUSCULOSKELETAL: OTHER CHANGES Annals of Pharmacotherpy. Vol. 33, Pg. 792, 1999.
5. Safety information of Levofloxacin
Hazard Codes:  Xn
Xn
Risk Statements: 22-42/43-68-20/21/22
R22:Harmful if swallowed.
R42/43:May cause sensitization by inhalation and skin contact.
R68:Possible risk of irreversible effects.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed.
Safety Statements: 26-36/37/39-36
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S36:Wear suitable protective clothing.
WGK Germany: 3
RTECS of Levofloxacin (CAS NO.100986-85-4): UU8815550
6. Uses of Levofloxacin
Levofloxacin is a broad spectrum antibiotic of the fluoroquinolone drug class. Levofloxacin is a chiral fluorinated carboxyquinolone. Investigation of ofloxacin, an older drug that is the racemic mixture, found that the l form [the (–)-(S) enantiomer] is more active. This specific component is levofloxacin.
7. Production of Levofloxacin
(1)2,3,4,5-Tetrafluorobenzoic acid can used to manufacture the Levofloxacin. And the condition is (S)-(+)-1-Amino-2-propanol and SOCl2 and so on. The detailed reaction is as follows:
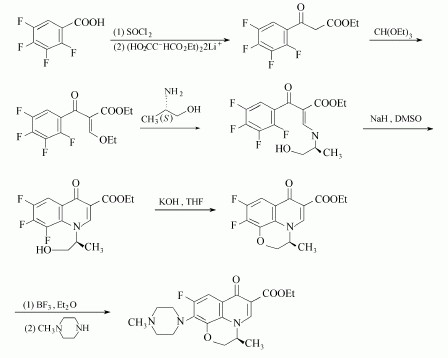
(2)2,3-DIFLUORO-6-NITROPHENOL can used to produce the Levofloxacin as follows:
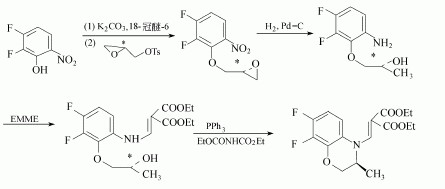
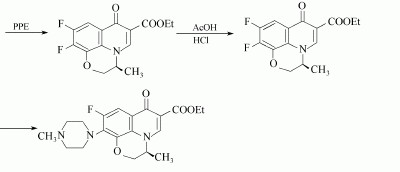
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View