-
Name
Metenolone
- EINECS 205-812-0
- CAS No. 153-00-4
- Article Data6
- CAS DataBase
- Density 1.084 g/cm3
- Solubility
- Melting Point 126-129°C(lit.)
- Formula C20H30O2
- Boiling Point 430.381 °C at 760 mmHg
- Molecular Weight 302.457
- Flash Point 183.597 °C
- Transport Information
- Appearance
- Safety
- Risk Codes R34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms 5α-Androst-1-en-3-one, 17β-hydroxy-1-methyl- (6CI,7CI,8CI);1-Methyl-5α-androst-1-en-17β-ol-3-one;1-Methyl-D1-androsten-17β-ol-3-one;17β-Hydroxy-1-methyl-5α-androst-1-en-3-one;Methenolon;Androst-1-en-3-one,17-hydroxy-1-methyl-, (5α,17β)-;
- PSA 37.30000
- LogP 4.12520
Synthetic route
-
-
4360-33-2
3-Aethylendioxy-1-methylen-5α-androstanol-(17β)

-
-
153-00-4
17β-hydroxy-1-methyl-5α-androst-1-en-3-one

| Conditions | Yield |
|---|---|
| (i) Zn-Cu, I2, CH2I2, Et2O, (ii) aq. H2SO4, MeOH; Multistep reaction; |

-
A
-
153-00-4
17β-hydroxy-1-methyl-5α-androst-1-en-3-one

-
F
-
1424-01-7
1-methyl-5α-androst-1-ene-3,17-dione

| Conditions | Yield |
|---|---|
| With Aspergillus niger In methanol at 26℃; for 288h; Microbiological reaction; |


-
-
153-00-4
17β-hydroxy-1-methyl-5α-androst-1-en-3-one

| Conditions | Yield |
|---|---|
| With Cunninghamella blakesleeana In methanol at 26℃; for 288h; Microbiological reaction; |
-
-
153-00-4
17β-hydroxy-1-methyl-5α-androst-1-en-3-one

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
127557-35-1
17β-(tert.-butyldimethylsilyloxy)-1-methyl-5α-androsta-1-en-3-one

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide for 24h; Ambient temperature; | 96% |
-
-
153-00-4
17β-hydroxy-1-methyl-5α-androst-1-en-3-one

-
-
96301-34-7
atamestane

| Conditions | Yield |
|---|---|
| With diphenyl diselenide; iodosylbenzene In toluene at 80℃; for 9h; | 51% |

| Conditions | Yield |
|---|---|
| With liver microsomes from Aroclor 1254-induced male Wistar rat In methanol; phosphate buffer at 37℃; pH=7.4; | A 24% B 28% |

-
-
153-00-4
17β-hydroxy-1-methyl-5α-androst-1-en-3-one

-
A
-
1232-57-1
1β-Methyl-4,5α-dihydro-testosteron (17β-Hydroxy-1β-methyl-5α-androstan-3-on)

-
B
-
1424-00-6
mesterolone

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); tributylzinc hydride In tetrahydrofuran for 1h; Heating; Yield given. Yields of byproduct given; |
-
-
153-00-4
17β-hydroxy-1-methyl-5α-androst-1-en-3-one

-
-
38631-89-9
(1S,5R,8R,9S,10S,13S,14S,17S)-1,10,13-Trimethyl-17-trimethylsilanyloxy-hexadecahydro-cyclopenta[a]phenanthren-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Tributylzinnhydrid, Azobisisobutyronitril / tetrahydrofuran / 1 h / Heating 2: hexamethyldisilazane / pyridine / 0.25 h View Scheme |
-
-
153-00-4
17β-hydroxy-1-methyl-5α-androst-1-en-3-one

-
-
38631-89-9
(1S,5S,8R,9S,10S,13S,14S,17S)-1,10,13-Trimethyl-17-trimethylsilanyloxy-hexadecahydro-cyclopenta[a]phenanthren-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Tributylzinnhydrid, Azobisisobutyronitril / tetrahydrofuran / 1 h / Heating 2: hexamethyldisilazane / pyridine / 0.25 h View Scheme |
-
-
153-00-4
17β-hydroxy-1-methyl-5α-androst-1-en-3-one

-
-
127557-29-3
1,4-dimethylandrosta-1,4-dien-3,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 96 percent / imidazole / dimethylformamide / 24 h / Ambient temperature 2: 1.) lithium diisopropylamide / 1.) THF, 0 deg, 15 min; 2.) THF, -78 deg C, 3 h; 3.) THF, -78 deg C, 15 min; 4.) r.t., 30 min 3: 87 percent / acetic acid / tetrahydrofuran; H2O / 72 h / 50 - 60 °C 4: 95 percent / dimethylaminopyridine / pyridine / 4 h / Ambient temperature 5: 31 percent / iodoxybenzene, diphenyl diselenide / toluene / 7 h / 80 °C 6: 92 percent / 3 percent potassium hydroxide / methanol / 4 h / Ambient temperature 7: 83 percent / chromium trioxide, conc. sulfuric acid / H2O; acetone / 0.25 h / Ambient temperature View Scheme |
-
-
153-00-4
17β-hydroxy-1-methyl-5α-androst-1-en-3-one

-
-
127557-37-3
17β-hydroxy-1,4α-dimethyl-5α-androst-1-en-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 96 percent / imidazole / dimethylformamide / 24 h / Ambient temperature 2: 1.) lithium diisopropylamide / 1.) THF, 0 deg, 15 min; 2.) THF, -78 deg C, 3 h; 3.) THF, -78 deg C, 15 min; 4.) r.t., 30 min 3: 87 percent / acetic acid / tetrahydrofuran; H2O / 72 h / 50 - 60 °C View Scheme |
-
-
153-00-4
17β-hydroxy-1-methyl-5α-androst-1-en-3-one

-
-
127557-41-9
1,4α-dimethyl-5α-androst-1-en-3,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 96 percent / imidazole / dimethylformamide / 24 h / Ambient temperature 2: 1.) lithium diisopropylamide / 1.) THF, 0 deg, 15 min; 2.) THF, -78 deg C, 3 h; 3.) THF, -78 deg C, 15 min; 4.) r.t., 30 min 3: 87 percent / acetic acid / tetrahydrofuran; H2O / 72 h / 50 - 60 °C 4: 84 percent / chromium trioxide, conc. sulfuric acid / H2O; acetone / 0.25 h / Ambient temperature View Scheme |
-
-
153-00-4
17β-hydroxy-1-methyl-5α-androst-1-en-3-one

-
-
127557-50-0
17β-hydroxy-1,2,4α-trimethyl-5α-androst-1-en-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 96 percent / imidazole / dimethylformamide / 24 h / Ambient temperature 2: 1.) lithium diisopropylamide / 1.) THF, 0 deg, 15 min; 2.) THF, -78 deg C, 3 h; 3.) THF, -78 deg C, 15 min; 4.) r.t., 30 min 3: 87 percent / acetic acid / tetrahydrofuran; H2O / 72 h / 50 - 60 °C 4: 95 percent / dimethylaminopyridine / pyridine / 4 h / Ambient temperature 5: 42 percent / various solvent(s); H2O; ethanol / 80 h / 110 °C 6: 70 percent / Raney nickel / acetone / 0.25 h / Heating 7: 90 percent / 3 percent potassium hydroxide / methanol / 4 h / Ambient temperature; 2 View Scheme |
-
-
153-00-4
17β-hydroxy-1-methyl-5α-androst-1-en-3-one

-
-
127557-40-8
17β-hydroxy-1,4-dimethylandrosta-1,4-dien-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 96 percent / imidazole / dimethylformamide / 24 h / Ambient temperature 2: 1.) lithium diisopropylamide / 1.) THF, 0 deg, 15 min; 2.) THF, -78 deg C, 3 h; 3.) THF, -78 deg C, 15 min; 4.) r.t., 30 min 3: 87 percent / acetic acid / tetrahydrofuran; H2O / 72 h / 50 - 60 °C 4: 95 percent / dimethylaminopyridine / pyridine / 4 h / Ambient temperature 5: 31 percent / iodoxybenzene, diphenyl diselenide / toluene / 7 h / 80 °C 6: 92 percent / 3 percent potassium hydroxide / methanol / 4 h / Ambient temperature View Scheme |
-
-
153-00-4
17β-hydroxy-1-methyl-5α-androst-1-en-3-one

-
-
127557-51-1
1,2,4α-trimethyl-5α-androst-1-en-3,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 96 percent / imidazole / dimethylformamide / 24 h / Ambient temperature 2: 1.) lithium diisopropylamide / 1.) THF, 0 deg, 15 min; 2.) THF, -78 deg C, 3 h; 3.) THF, -78 deg C, 15 min; 4.) r.t., 30 min 3: 87 percent / acetic acid / tetrahydrofuran; H2O / 72 h / 50 - 60 °C 4: 95 percent / dimethylaminopyridine / pyridine / 4 h / Ambient temperature 5: 42 percent / various solvent(s); H2O; ethanol / 80 h / 110 °C 6: 70 percent / Raney nickel / acetone / 0.25 h / Heating 7: 90 percent / 3 percent potassium hydroxide / methanol / 4 h / Ambient temperature; 2 8: 83 percent / chromium trioxide, conc. sulfuric acid / H2O; acetone / 0.25 h / Ambient temperature View Scheme |
-
-
153-00-4
17β-hydroxy-1-methyl-5α-androst-1-en-3-one

-
-
127557-49-7
17β-hydroxy-1,2,4-trimethylandrosta-1,4-dien-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 96 percent / imidazole / dimethylformamide / 24 h / Ambient temperature 2: 1.) lithium diisopropylamide / 1.) THF, 0 deg, 15 min; 2.) THF, -78 deg C, 3 h; 3.) THF, -78 deg C, 15 min; 4.) r.t., 30 min 3: 87 percent / acetic acid / tetrahydrofuran; H2O / 72 h / 50 - 60 °C 4: 95 percent / dimethylaminopyridine / pyridine / 4 h / Ambient temperature 5: 42 percent / various solvent(s); H2O; ethanol / 80 h / 110 °C 6: 70 percent / Raney nickel / acetone / 0.25 h / Heating 7: 33 percent / iodoxybenzene, diphenyl diselenide / toluene / 80 °C 8: 90 percent / 3 percent potassium hydroxide / methanol / 4 h / Ambient temperature View Scheme |
-
-
153-00-4
17β-hydroxy-1-methyl-5α-androst-1-en-3-one

-
-
127557-31-7
1,2,4-trimethylandrosta-1,4-dien-3,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: 96 percent / imidazole / dimethylformamide / 24 h / Ambient temperature 2: 1.) lithium diisopropylamide / 1.) THF, 0 deg, 15 min; 2.) THF, -78 deg C, 3 h; 3.) THF, -78 deg C, 15 min; 4.) r.t., 30 min 3: 87 percent / acetic acid / tetrahydrofuran; H2O / 72 h / 50 - 60 °C 4: 95 percent / dimethylaminopyridine / pyridine / 4 h / Ambient temperature 5: 42 percent / various solvent(s); H2O; ethanol / 80 h / 110 °C 6: 70 percent / Raney nickel / acetone / 0.25 h / Heating 7: 33 percent / iodoxybenzene, diphenyl diselenide / toluene / 80 °C 8: 90 percent / 3 percent potassium hydroxide / methanol / 4 h / Ambient temperature 9: 82 percent / chromium trioxide, conc. sulfuric acid / H2O; acetone / 0.25 h / Ambient temperature View Scheme |
-
-
153-00-4
17β-hydroxy-1-methyl-5α-androst-1-en-3-one

-
-
127557-38-4
17β-acetyloxy-1,4α-dimethyl-5α-androst-1-en-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 96 percent / imidazole / dimethylformamide / 24 h / Ambient temperature 2: 1.) lithium diisopropylamide / 1.) THF, 0 deg, 15 min; 2.) THF, -78 deg C, 3 h; 3.) THF, -78 deg C, 15 min; 4.) r.t., 30 min 3: 87 percent / acetic acid / tetrahydrofuran; H2O / 72 h / 50 - 60 °C 4: 95 percent / dimethylaminopyridine / pyridine / 4 h / Ambient temperature View Scheme |
-
-
153-00-4
17β-hydroxy-1-methyl-5α-androst-1-en-3-one

-
-
127557-47-5
17β-acetyloxy-1,2,4α-trimethyl-5α-androst-1-en-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 96 percent / imidazole / dimethylformamide / 24 h / Ambient temperature 2: 1.) lithium diisopropylamide / 1.) THF, 0 deg, 15 min; 2.) THF, -78 deg C, 3 h; 3.) THF, -78 deg C, 15 min; 4.) r.t., 30 min 3: 87 percent / acetic acid / tetrahydrofuran; H2O / 72 h / 50 - 60 °C 4: 95 percent / dimethylaminopyridine / pyridine / 4 h / Ambient temperature 5: 42 percent / various solvent(s); H2O; ethanol / 80 h / 110 °C 6: 70 percent / Raney nickel / acetone / 0.25 h / Heating View Scheme |
-
-
153-00-4
17β-hydroxy-1-methyl-5α-androst-1-en-3-one

-
-
127557-39-5
17β-acetyloxy-1,4-dimethylandrosta-1,4-dien-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 96 percent / imidazole / dimethylformamide / 24 h / Ambient temperature 2: 1.) lithium diisopropylamide / 1.) THF, 0 deg, 15 min; 2.) THF, -78 deg C, 3 h; 3.) THF, -78 deg C, 15 min; 4.) r.t., 30 min 3: 87 percent / acetic acid / tetrahydrofuran; H2O / 72 h / 50 - 60 °C 4: 95 percent / dimethylaminopyridine / pyridine / 4 h / Ambient temperature 5: 31 percent / iodoxybenzene, diphenyl diselenide / toluene / 7 h / 80 °C View Scheme |
-
-
153-00-4
17β-hydroxy-1-methyl-5α-androst-1-en-3-one

-
-
127557-48-6
17β-acetyloxy-1,2,4-trimethylandrosta-1,4-dien-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 96 percent / imidazole / dimethylformamide / 24 h / Ambient temperature 2: 1.) lithium diisopropylamide / 1.) THF, 0 deg, 15 min; 2.) THF, -78 deg C, 3 h; 3.) THF, -78 deg C, 15 min; 4.) r.t., 30 min 3: 87 percent / acetic acid / tetrahydrofuran; H2O / 72 h / 50 - 60 °C 4: 95 percent / dimethylaminopyridine / pyridine / 4 h / Ambient temperature 5: 42 percent / various solvent(s); H2O; ethanol / 80 h / 110 °C 6: 70 percent / Raney nickel / acetone / 0.25 h / Heating 7: 33 percent / iodoxybenzene, diphenyl diselenide / toluene / 80 °C View Scheme |
-
-
153-00-4
17β-hydroxy-1-methyl-5α-androst-1-en-3-one

-
-
127557-36-2
17β-(tert.-butyldimethylsilyloxy)-1,4α-dimethyl-5α-androsta-1-en-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 96 percent / imidazole / dimethylformamide / 24 h / Ambient temperature 2: 1.) lithium diisopropylamide / 1.) THF, 0 deg, 15 min; 2.) THF, -78 deg C, 3 h; 3.) THF, -78 deg C, 15 min; 4.) r.t., 30 min View Scheme |
-
-
153-00-4
17β-hydroxy-1-methyl-5α-androst-1-en-3-one

-
-
127557-46-4
17β-acetyloxy-1,4α-dimethyl-2-<(phenylthio)methyl>-5α-androst-1-en-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 96 percent / imidazole / dimethylformamide / 24 h / Ambient temperature 2: 1.) lithium diisopropylamide / 1.) THF, 0 deg, 15 min; 2.) THF, -78 deg C, 3 h; 3.) THF, -78 deg C, 15 min; 4.) r.t., 30 min 3: 87 percent / acetic acid / tetrahydrofuran; H2O / 72 h / 50 - 60 °C 4: 95 percent / dimethylaminopyridine / pyridine / 4 h / Ambient temperature 5: 42 percent / various solvent(s); H2O; ethanol / 80 h / 110 °C View Scheme |
-
-
24589-78-4
N-methyl-N-trimethylsilyl-2,2,2-trifluoroacetamide

-
-
153-00-4
17β-hydroxy-1-methyl-5α-androst-1-en-3-one

-
-
136693-28-2
C26H46O2Si2

| Conditions | Yield |
|---|---|
| With ammonium iodide; 2-hydroxyethanethiol at 60℃; for 0.333333h; | |
| With ammonium iodide; ethanethiol In acetonitrile at 80℃; for 0.5h; |
Metenolone Specification
1. Introduction of Methenolone
Methenolone is one kind of white or almost white crystalline powder. The IUPAC Name of this chemical is (5S,8R,9S,10S,13S,14S,17S)-17-hydroxy-1,10,13-trimethyl-4,5,6,7,8,9,11,
12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one. Besides, Methenolone belongs to Steroids; Steroid and Hormone; Finished Steroid and Hormone. What's more, its systematic name is (5α,17β)-17-Hydroxy-1-methylandrost-1-en-3-one. Its classification codes are: (1)Anabolic Agents; (2)Hormones; (3)Hormones, Hormone Substitutes, and Hormone Antagonists.
This chemical is a synthetic steroid that has been used for its anabolic action. It is supplied as the acetate ester for oral administration and as the enanthate ester for intramuscular injection.
2. Properties of Methenolone
Physical properties about Methenolone are:
(1)ACD/LogP: 3.717; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 3.72; (4)ACD/LogD (pH 7.4): 3.72; (5)ACD/BCF (pH 5.5): 393.28; (6)ACD/BCF (pH 7.4): 393.28; (7)ACD/KOC (pH 5.5): 2505.49; (8)ACD/KOC (pH 7.4): 2505.49; (9)#H bond acceptors: 2; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 37.3 Å2; (13)Index of Refraction: 1.542; (14)Molar Refractivity: 87.803 cm3; (15)Molar Volume: 278.897 cm3; (16)Polarizability: 34.808×10-24cm3; (17)Surface Tension: 40.06 dyne/cm; (18)Density: 1.084 g/cm3; (19)Flash Point: 183.597 °C; (20)Enthalpy of Vaporization: 79.187 kJ/mol; (21)Boiling Point: 430.381 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25°C.
3. Structure Descriptors of Methenolone
You can still convert the following datas into molecular structure:
(1)SMILES: O=C2\C=C(\C)[C@@]3([C@H]1CC[C@@]4([C@@H](O)CC[C@H]4[C@@H]1CC[C@H]3C2)C)C
(2)Std. InChI: InChI=1S/C20H30O2/c1-12-10-14(21)11-13-4-5-15-16-6-7-18(22)19(16,2)9-8-17(15)20(12,13)3/h10,13,15-18,22H,4-9,11H2,1-3H3/t13-,15-,16-,17-,18-,19-,20-/m0/s1
(3)Std. InChIKey: ANJQEDFWRSLVBR-VHUDCFPWSA-N
(4)Canonical SMILES : CC1=CC(=O)CC2C1(C3CCC4(C(C3CC2)CCC4O)C)C
(5)Isomeric SMILES: CC1=CC(=O)C[C@H]2[C@]1([C@H]3CC[C@]4([C@H]([C@@H]3CC2)CC[C@@H]4O)C)C
4. Physical Properties of Methenolone
| Physical Property | Value | Units | Temp (deg C) | Source |
|---|---|---|---|---|
| log P (octanol-water) | 3.690 | (none) | EST | |
| Atmospheric OH Rate Constant | 1.15E-10 | cm3/molecule-sec | 25 | EST |
5. Uses of Methenolone
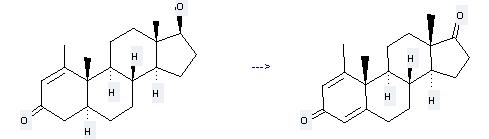
Methenolone can be used to produce 1-methylandrosta-1,4-dien-3,17-dione at the temperature of 80 °C. It will need reagents iodoxybenzene, diphenyl diselenide and solvent toluene with the reaction time of 9 hours. The yield is about 51%.
Related Products
- Metenolone
- 153004-31-0
- 1530-04-7
- 153012-08-9
- 153012-37-4
- 15301-54-9
- 15301-69-6
- 15302-16-6
- 1530-32-1
- 153034-78-7
- 153034-80-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View