-
Name
METYRAPONE
- EINECS 200-206-2
- CAS No. 54-36-4
- Article Data8
- CAS DataBase
- Density 1.12g/cm3
- Solubility
-
Melting Point
52-55 °C(lit.)
- Formula C14H14 N2 O
- Boiling Point 384.4°Cat760mmHg
- Molecular Weight 226.278
- Flash Point 189.3°C
- Transport Information
- Appearance
- Safety Poison by intraperitoneal route. Moderately toxic by ingestion. An experimental teratogen. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
- Risk Codes 22-36/37/38
-
Molecular Structure
- Hazard Symbols Xn
- Synonyms 1-Propanone,2-methyl-1,2-di-3-pyridyl- (6CI,8CI); 1,2-Bis(3-pyridyl)-2-methyl-1-propanone;2-Methyl-1,2-bis(3-pyridyl)-1-propanone; 2-Methyl-1,2-di(b-pyridyl)-1-propanone;2-Methyl-1,2-di-3-pyridyl-1-propanone; Mepyrapone; Methapyrapone;Methbipyranone; Methopirapone; Methopyrapone; Methopyrinine; Methopyrone;Metopiron; Metopirone; Metopyrone; Metroprione; Metyrapon; Metyrapone; NSC25265; Su 4885
- PSA 42.85000
- LogP 2.63710
Synthetic route

-
A
-
54-36-4
metyrapone

-
B
-
33977-49-0
3,3-di-pyridin-3-yl-butan-2-one

-
C
-
96953-46-7
3,3'-(1,2-bis-methylene-ethane-1,2-diyl)-bis-pyridine

| Conditions | Yield |
|---|---|
| With sulfuric acid for 70h; Further byproducts given; | A 22% B 38% C 13% D 14% |

-
-
4989-59-7
2,3-di-3-pyridyl-2,3-butanediol

-
A
-
54-36-4
metyrapone

-
B
-
33977-49-0
3,3-di-pyridin-3-yl-butan-2-one

| Conditions | Yield |
|---|---|
| With sulfuric acid |

-
A
-
54-36-4
metyrapone

-
B
-
33977-49-0
3,3-di-pyridin-3-yl-butan-2-one

| Conditions | Yield |
|---|---|
| With sulfuric acid at 23℃; Product distribution; Further Variations:; Reagents; |


-
A
-
54-36-4
metyrapone

-
B
-
96953-46-7
3,3'-(1,2-bis-methylene-ethane-1,2-diyl)-bis-pyridine

| Conditions | Yield |
|---|---|
| With sulfuric acid at 23℃; Product distribution; Further Variations:; Reagents; Temperatures; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: isopropyl alcohol; acetic acid / Einwirkung von UV-Licht 2: concentrated H2SO4 View Scheme |

| Conditions | Yield |
|---|---|
| With tri-tert-butyl phosphine; palladium diacetate; sodium t-butanolate In tetrahydrofuran for 3.5h; Inert atmosphere; Reflux; |

-
-
75-05-8
acetonitrile

-
A
-
54-36-4
metyrapone

-
B
-
55124-54-4
bis(acetonitrile)bis(2,2'-bipyridine)ruthenium(II) hexafluorophosphate

| Conditions | Yield |
|---|---|
| for 1h; Kinetics; Time; UV-irradiation; |


| Conditions | Yield |
|---|---|
| for 0.0166667h; Kinetics; Time; UV-irradiation; |

-
-
54-36-4
metyrapone

-
-
17159-42-1, 31337-87-8, 162936-53-0
Metyrapol

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate Reduction; | 100% |
| With sodium tetrahydroborate In methanol Reduction; | |
| With human liver dihydrodiol dehydrogenase 4; NADPH In phosphate buffer at 25℃; pH=6.0; Enzyme kinetics; Further Variations:; Reagents; |
-
-
54-36-4
metyrapone

| Conditions | Yield |
|---|---|
| With (R)-3,3'-bis(2,4,6-triisopropylphenyl)binol phosphoric acid; [4,4’-bis(1,1-dimethylethyl)-2,2’-bipyridine-N1,N1‘]bis [3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-N]phenyl-C]iridium(III) hexafluorophosphate In 1,4-dioxane for 14h; Minisci Aromatic Substitution; Irradiation; enantioselective reaction; | 70% |
-
-
54-36-4
metyrapone

-
-
745783-97-5
triethyl(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)silane

| Conditions | Yield |
|---|---|
| With 1,2-dimethoxyethane; potassium hexamethylsilazane for 3h; Inert atmosphere; regioselective reaction; | 54% |

| Conditions | Yield |
|---|---|
| With tert-Butyl peroxybenzoate In dichloromethane at 20℃; for 12h; Catalytic behavior; Irradiation; Inert atmosphere; | 46% |
-
-
17084-13-8
potassium hexafluorophosphate

-
-
54-36-4
metyrapone

| Conditions | Yield |
|---|---|
| Stage #1: metyrapone; bis(2,2'-bipyridine)dichlororuthenium(II) dihydrate In water at 80℃; Inert atmosphere; Stage #2: potassium hexafluorophosphate In water | 38% |

-
-
54-36-4
metyrapone

-
A
-
15825-89-5
3-(1-methylethenyl)pyridine

-
B
-
6304-18-3
3-isopropylpyridine

-
C
-
59-67-6
nicotinic acid

-
D
-
15031-77-3
2-(3-pyridinyl)-2-propanol

-
F
-
37388-00-4
2,3-di(3-pyridyl)-2,3-dimethylbutane

| Conditions | Yield |
|---|---|
| With 1-dodecylthiol In cyclohexane Quantum yield; Product distribution; Irradiation; | A 4% B 6% C 15% D 15% E n/a F 35% |

-
-
54-36-4
metyrapone

-
A
-
15825-89-5
3-(1-methylethenyl)pyridine

-
B
-
6304-18-3
3-isopropylpyridine

-
C
-
15031-77-3
2-(3-pyridinyl)-2-propanol

-
D
-
37388-00-4
2,3-di(3-pyridyl)-2,3-dimethylbutane

| Conditions | Yield |
|---|---|
| In methanol for 6h; Irradiation; | A 4% B 2% C 2% D 35% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) for 40h; Product distribution; Mechanism; Irradiation; also in MeCN solution; | A 30% B 11% C 6% D 3% E 4% |

-
-
54-36-4
metyrapone

-
B
-
71539-51-0
2-Methyl-1-<3'-(1'-oxopyridyl)>-2-(3''-pyridyl)-propan-1-one

-
C
-
63473-06-3
2-Methyl-2-<3''-(1''-oxopyridyl)>-1-(3'-pyridyl)-propan-1-one

-
D
-
73292-52-1
2-Methyl-1,2-bis-3-(1-oxopyridyl)propan-1-one

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In chloroform for 0.0833333h; -10 deg C to r.t.; Yield given. Yields of byproduct given; | |
| With 3-chloro-benzenecarboperoxoic acid In chloroform 1.) -10 deg C to r.t., 2.) r.t., 5 min; Yield given. Yields of byproduct given; |

-
-
54-36-4
metyrapone

| Conditions | Yield |
|---|---|
| 1.) H2O, irrad., 6 h, 2.) heating; Yield given. Multistep reaction; |
-
-
54-36-4
metyrapone

| Conditions | Yield |
|---|---|
| With (S)-3,3'-bis(2,4,6-tri-iso-propylphenyl)-1,1'-binaphthyl-2,2'-diyl hydrogenphosphate; triphenylphosphine; sodium iodide In 1,4-dioxane at 20℃; for 20h; Schlenk technique; Inert atmosphere; Irradiation; Overall yield = 55 %; | A n/a B n/a |
| With (R)-3,3'-bis(2,4,6-triisopropylphenyl)binol phosphoric acid; triphenylphosphine; sodium iodide In 1,4-dioxane at 20℃; for 15h; Irradiation; Overall yield = 55 %; enantioselective reaction; | A n/a B n/a |


| Conditions | Yield |
|---|---|
| With rose bengal In dimethyl sulfoxide at 20℃; Irradiation; Overall yield = 33 percent; |

Metyrapone Chemical Properties
Product Name: Metyrapone (CAS NO.54-36-4)
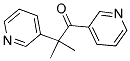
Molecular Formula: C14H14N2O
Molecular Weight: 226.27g/mol
Mol File: 54-36-4.mol
Einecs: 200-206-2
Melting Point: 52-55 °C(lit.)
Boiling point: 384.4 °C at 760 mmHg
Flash Point: 189.3 °C
Density: 1.12 g/cm3
Surface Tension: 45.4 dyne/cm
Enthalpy of Vaporization: 63.3 kJ/mol
Vapour Pressure: 4.11E-06 mmHg at 25°C
XLogP3-AA: 2
H-Bond Donor: 0
H-Bond Acceptor: 3
Structure Descriptors of Metyrapone (CAS NO.54-36-4):
IUPAC Name: 2-methyl-1,2-dipyridin-3-ylpropan-1-one
Canonical SMILES: CC(C)(C1=CN=CC=C1)C(=O)C2=CN=CC=C2
InChI: InChI=1S/C14H14N2O/c1-14(2,12-6-4-8-16-10-12)13(17)11-5-3-7-15-9-11/h3-10H,1-2H3
InChIKey: FJLBFSROUSIWMA-UHFFFAOYSA-N
Product Categories: Cytochrome P450 isozyme
Metyrapone Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | subcutaneous | 531mg/kg (531mg/kg) | Cesko-Slovenska Farmacie. Vol. 26, Pg. 140, 1977. | |
| mouse | LDLo | intraperitoneal | 300mg/kg (300mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 146, Pg. 395, 1964. | |
| rat | LD50 | oral | 521mg/kg (521mg/kg) | Drugs in Japan Vol. 6, Pg. 827, 1982. |
Metyrapone Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion. An experimental teratogen. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
Safety Information of Metyrapone (CAS NO.54-36-4):
Hazard Codes: Xn
Risk Statements: 22-36/37/38
22: Harmful if swallowed
36: Irritating to the eyes
37: Irritating to the respiratory system
38: Irritating to the skin
Safety Statements: 26
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
Metyrapone Specification
Metyrapone ,its CAS NO. is 54-36-4,the synonyms is 1,2-Di-3-pyridyl-2-methyl-1-propanone ; 1-Propanone, 1,2-di-3-pyridyl-2-methyl- ; 1-Propanone, 2-methyl-1,2-di-3-pyridinyl- ; 2-Methyl-1,2-bis(3-pyridyl)-1-propanone ; 2-Methyl-1,2-di-3-pyridyl-1-propanone ; EINECS 200-206-2 ; HSDB 2500 ; Methapyrapone ; Metyraponum ; NSC 25265 ; UNII-ZS9KD92H6V .
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View