-
Name
PHENYLUREA
- EINECS 200-576-5
- CAS No. 64-10-8
- Article Data204
- CAS DataBase
- Density 1,302 g/cm3
-
Solubility
Stability
- Stable. Incompatible with strong oxidizing agents.
Toxicology
- Harmful if swallowed. May be harmful if inhaled orabsorbed through the skin. Toxicology not fully investigated.
- Risk Codes R22
-
Molecular Structure
-
Hazard Symbols

- Synonyms Urea,phenyl- (8CI,9CI); 1-Phenylurea; Ethyl 5-nitro-1H-indole-3-carboxylate;Monophenylurea; N-Phenylurea; NSC 2781; Phenylcarbamide; Phenylurea;Stabilisator VH; Stabilizer VH; VH
- PSA 55.12000
- LogP 1.95050
x.
N-Phenylurea Chemical Properties
IUPAC Name: Phenylurea
Product Name: Phenylurea
The MF of Phenylurea (CAS NO.64-10-8) is C7H8N2O.
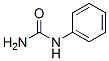
The MW of Phenylurea (CAS NO.64-10-8) is 136.15.
Synonyms of Phenylurea (CAS NO.64-10-8): 1-Phenylurea ; Monophenylurea ; N-Phenylurea ; Phenylcarbamide ; Phenylurea ; Stabilisator VH ; Stabilizer VH ; Urea, phenyl-
Stability: Stable. Incompatible with strong oxidizing agents
Solubility in H2O: 10 mg/mL, clear
Apperance: off-white powder
Index of Refraction: 1.638
EINECS: 200-576-5
Density: 1.241 g/ml
Flash Point: 97.7 °C
Boiling Point: 238 °C
Melting Point: 145-147 °C
Merck: 14,7319
BRN: 1934615
N-Phenylurea Uses
Phenylurea (CAS NO.64-10-8) is used in organic synthesis.
N-Phenylurea Production
Organic amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx). Contains any of several related compounds (Diuron, Fenuron, Linuron, Neburon, Siduron, Monuron) formally derived from urea.
N-Phenylurea Toxicity Data With Reference
| 1. | orl-rat LD50:2000 mg/kg | IIFBA4 Izvestiya na Instituta po Fiziologiya, Bulgarska Akademiya na Naukite. 12 (1969),195. | ||
| 2. | orl-mus LD50:1580 mg/kg | IIFBA4 Izvestiya na Instituta po Fiziologiya, Bulgarska Akademiya na Naukite. 12 (1969),195. | ||
| 3. | ipr-mus LD50:1060 mg/kg | IIFBA4 Izvestiya na Instituta po Fiziologiya, Bulgarska Akademiya na Naukite. 12 (1969),195. |
N-Phenylurea Consensus Reports
Reported in EPA TSCA Inventory.
N-Phenylurea Safety Profile
Moderately toxic by ingestion and intraperitoneal routes. When heated to decomposition it emits toxic fumes of NOx.Safety information of Phenylurea (CAS NO.64-10-8):
Hazard Codes  Xn
Xn
Risk Statements
22 Harmful if swallowed
Safety Statements
22 Do not breathe dust
36/37 Wear suitable protective clothing and gloves
WGK Germany 3
RTECS YU0650000
N-Phenylurea Specification
A solid or liquid absorbed on a dry carrier. A wettable powder. Contains any of several related products (Diuron, Fenuron, Linuron, Monuron, Neburon, Siduron) formally derived from urea. Toxic by inhalation, skin absorption, or ingestion. Obtain the technical name of the specific pesticide from the shipping papers and contact CHEMTREC, 800-424-9300 for response information.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View