-
Name
Phenelzine
- EINECS 200-117-9
- CAS No. 51-71-8
- Density 1.01 g/cm3
- Solubility
- Melting Point 25°C
- Formula C8H12 N2
- Boiling Point 281.4 °C at 760 mmHg
- Molecular Weight 136.197
- Flash Point 143.7 °C
- Transport Information
- Appearance
- Safety Poison by ingestion, intraperitoneal, and subcutaneous routes. Human systemic effects by ingestion: ataxia, somnolence. An experimental teratogen. Experimental reproductive effects. Mutation data reported. Used as an antidepressant. When heated to decomposition it emits toxic fumes of NOx.
- Risk Codes
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Hydrazine,phenethyl- (6CI,8CI); 1-(2-Phenethyl)hydrazine; 1-(2-Phenylethyl)hydrazine;1-Hydrazino-2-phenylethane; 2-Phenylethylhydrazine; Phenalzine; Phenelzine;Phenethylhydrazine; Phenylethylhydrazine; W 1544; b-Phenylethylhydrazine
- PSA 38.05000
- LogP 1.78360
Phenelzine Chemical Properties
Chemistry informtion about 2-Phenylethylhydrazine (CAS NO.51-71-8) is:
IUPAC Name: Phenethylhydrazine
Synonyms: Asinex-Reag Bas 07650440 ; Beta-Phenylethylhydrazine ; 2-Phenelzine ; (2-Phenyl-Ethyl)-Hydrazine ; Phenethyl Hydrazine ; Phenelzine ; (2-Phenylethyl)-Hydrazin ; 1-(2-Phenylethyl)Hydrazine
MF: C8H12N2
MW: 136.19
EINECS: 200-117-9
Density: 1.01 g/cm3
Flash Point: 143.7 °C
Boiling Point: 281.4 °C at 760 mmHg
Vapour Pressure: 0.00358 mmHg at 25°C
Enthalpy of Vaporization: 52.02 kJ/mol
Following is the molecular structure of 2-Phenylethylhydrazine (CAS NO.51-71-8) is:
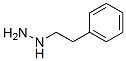
Phenelzine Uses
2-Phenylethylhydrazine (CAS NO.51-71-8) is used primarily in the treatment of major depressive disorder (MDD). Patients with depressive symptomology characterized as "atypical", "nonendogenous", and/or "neurotic", have been reported to respond particularly well to phenelzine. The medication has also been found to be useful in patients who do not respond favorably to first and second-line treatments for depression, or are said to be "treatment-resistant". In addition to being a recognized treatment for major depressive disorder, it has been found in studies to be effective in treating dysthymia, bipolar depression (BD), panic disorder (PD), social anxiety disorder (SAD), bulimia, and post-traumatic stress disorder (PTSD).
Phenelzine Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| child | TDLo | oral | 7500ug/kg (7.5mg/kg) | behavioral: ataxia behavioral: somnolence (general depressed activity) | American Journal of Diseases of Children. Vol. 130, Pg. 507, 1976. |
| mouse | LD50 | intraperitoneal | 135mg/kg (135mg/kg) | Farmakologiya i Toksikologiya Vol. 32, Pg. 526, 1969. | |
| mouse | LD50 | oral | 130mg/kg (130mg/kg) | Biochemical Pharmacology. Vol. 17, Pg. 369, 1968. | |
| mouse | LD50 | subcutaneous | 122mg/kg (122mg/kg) | Farmakologiya i Toksikologiya Vol. 37, Pg. 651, 1974. | |
| women | LDLo | oral | 30mg/kg (30mg/kg) | lungs, thorax, or respiration: acute pulmonary edema liver: fatty liver degeration | Journal of Analytical Toxicology. Vol. 19, Pg. 265, 1995. |
| women | TDLo | oral | 39600ug/kg/44 (39.6mg/kg) | behavioral: "hallucinations, distorted perceptions" behavioral: toxic psychosis behavioral: muscle contraction or spasticity) | Journal of Clinical Psychiatry. Vol. 48, Pg. 340, 1987. |
Phenelzine Safety Profile
Poison by ingestion, intraperitoneal, and subcutaneous routes. Human systemic effects by ingestion: ataxia, somnolence. An experimental teratogen. Experimental reproductive effects. Mutation data reported. Used as an antidepressant. When heated to decomposition it emits toxic fumes of NOx.
Hazard Codes:
 Xi
Xi
Phenelzine Specification
2-Phenylethylhydrazine (CAS NO.51-71-8) is an irreversible and nonselective and monoamine oxidase inhibitor (MAOI) of the hydrazine chemical class which is used as an antidepressant and anxiolytic. Along with tranylcypromine and isocarboxazid, phenelzine is one of the few nonselective MAOIs still in widespread clinical use. 2-Phenylethylhydrazine and the other MAOIs are typically considered to be significantly more effective against clinical depression in comparison to more mainstream antidepressants like the selective serotonin reuptake inhibitors (SSRIs), but are usually reserved only as a last resort due to their prominent side effects and potentially hazardous food and drug interactions. Notably, phenelzine has shown unique efficacy in treating anxiety disorders, likely due to its actions on GABA concentrations.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View