-
Name
(3-BROMOPROPYL)TRIPHENYLPHOSPHONIUM BROMIDE
- EINECS 222-770-9
- CAS No. 3607-17-8
- Article Data26
- CAS DataBase
- Density
- Solubility
- Melting Point 228-230 °C(lit.)
- Formula C21H21Br2P
- Boiling Point
- Molecular Weight 464.179
- Flash Point
- Transport Information
- Appearance white to off-white crystalline powder
- Safety 26-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms (3-Bromopropyl)triphenylphosphoniumbromide (6CI,7CI);Phosphonium, (3-bromopropyl)triphenyl-, bromide (8CI,9CI);NSC 84074;
- PSA 13.59000
- LogP 1.76950
Phosphonium,(3-bromopropyl)triphenyl-, bromide (1:1) Specification
The Phosphonium,(3-bromopropyl)triphenyl-, bromide (1:1), with the CAS registry number 3607-17-8 and EINECS registry number 222-770-9, has the systematic name of (3-bromopropyl)(triphenyl)phosphonium bromide. It is a kind of white to off-white crystalline powder, and belongs to the following product categories: Phosphonium Compounds; Synthetic Organic Chemistry; Wittig & Horner-Emmons Reaction; Wittig Reaction; C-C Bond Formation; Olefination; Wittig Reagents. And the molecular formula of the chemical is C21H21Br2P.
The characteristics of Phosphonium,(3-bromopropyl)triphenyl-, bromide (1:1) are as followings: (1)H-Bond Donor 0; (2)H-Bond Acceptor 1; (3)Rotatable Bond Count 6; (4)Exact Mass 463.972716; (5)MonoIsotopic Mass 461.974762; (6)Topological Polar Surface Area 0; (7)Heavy Atom Count 24; (8)Formal Charge 0; (9)Complexity 279; (10)Isotope Atom Count 0; (11)Defined Atom StereoCenter Count 0; (12)Undefined Atom StereoCenter Count 0; (13)Defined Bond StereoCenter Count 0; (14)Undefined Bond StereoCenter Count 0; (15)Covalently-Bonded Unit Count 2.
Preparation of Phosphonium,(3-bromopropyl)triphenyl-, bromide (1:1): This chemical can be prepared by triphenylphosphane and 1,3-dibromo-propane. The reaction will need the menstruum toluene. The reaction time is 5 hours with heating, and the yield is about 66%.

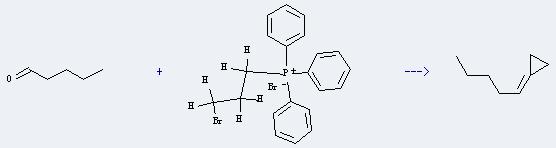
Uses of Phosphonium,(3-bromopropyl)triphenyl-, bromide (1:1): It can react with pentanal to produce n-pentylidene cyclopropane. This reaction will need reagent 50% NaH, and the yield is about 40%.
You should be cautious while dealing with this chemical. It irritates to eyes, respiratory system and skin. Therefore, you had better take the following instructions: Wear suitable gloves and eye/face protection, and if in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice.
Addtionally, the following datas could be converted into the molecular structure:
(1)SMILES: [Br-].BrCCC[P+](c1ccccc1)(c2ccccc2)c3ccccc3
(2)InChI: InChI=1/C21H21BrP.BrH/c22-17-10-18-23(19-11-4-1-5-12-19,20-13-6-2-7-14-20)21-15-8-3-9-16-21;/h1-9,11-16H,10,17-18H2;1H/q+1;/p-1
(3)InChIKey: ZAHUZZUGJRPGKW-REWHXWOFAA
Related Products
- Phosphonium iodide
- Phosphonium, triphenyl[3-(trimethylsilyl)-2-propyn-1-yl]-, bromide (1:1)
- Phosphonium,(1-methylpropyl)triphenyl-, bromide (1:1)
- Phosphonium,(2-carboxyethyl)triphenyl-, chloride (1:1)
- Phosphonium,(2-ethoxy-2-oxoethyl)triphenyl-, chloride (1:1)
- Phosphonium,(2-hydroxyethyl)triphenyl-, bromide (1:1)
- Phosphonium,(2-hydroxyethyl)triphenyl-, chloride (1:1)
- Phosphonium,(3-bromopropyl)triphenyl-, bromide (1:1)
- Phosphonium,(4-ethoxy-2,4-dioxobutyl)triphenyl-, chloride (1:1)
- Phosphonium,(4-ethoxy-4-oxobutyl)triphenyl-, bromide (1:1)
- 36072-16-9
- 36073-93-5
- 36075-68-0
- 360762-48-7
- 36076-26-3
- 3607-78-1
- 360791-53-3
- 36082-50-5
- 36083-56-4
- 36083-80-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View