-
Name
Prazosin
- EINECS 242-885-8
- CAS No. 19216-56-9
- Article Data19
- CAS DataBase
- Density 1.353 g/cm3
- Solubility 3.2mg/L(22.5 oC)
- Melting Point 278-280°C
- Formula C19H21N5O4
- Boiling Point 638.366 °C at 760 mmHg
- Molecular Weight 383.407
- Flash Point 339.871 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Piperazine,1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanylcarbonyl)- (9CI);Piperazine, 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furoyl)- (8CI);4-(4-Amino-6,7-dimethoxyquinazolin-2-yl)piperazinyl 2-furyl ketone;Furazosin;Lentopres;
- PSA 106.95000
- LogP 3.17070
Prazosin Chemical Properties
IUPAC Name: [4-(4-Amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl]-(furan-2-yl)methanone
The MF of Prazosin (CAS NO.19216-56-9) is C19H21N5O4.
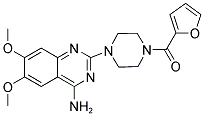
The MW of Prazosin (CAS NO.19216-56-9) is 383.4.
Synonyms of Prazosin (CAS NO.19216-56-9): [4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl](furan-2-yl)methanone ; 1-(4-Amino-6, 7-dimethoxy-2-quinazolinyl)-4-(2-furoyl)piperazine monohydrochloride
Apperance: Crystallization
Index of Refraction: 1.651
EINECS: 242-885-8
Density: 1.352 g/ml
Flash Point: 339.9 °C
Boiling Point: 638.4 °C
Melting Point: 278-280 °C
Prazosin Uses
Prazosin (CAS NO.19216-56-9) is orally active and has a minimal effect on cardiac function due to its alpha-1 receptor selectivity.it is a second-line choice for the treatment of high blood pressure.Prazosin is also useful in treating urinary hesitancy associated with prostatic hyperplasia by blocking alpha-1 receptors, which control constriction of both the prostate and ureters.This medication has shown to be effective in treating severe nightmares in children, associated with PTSD symptoms.
Prazosin Production
3,4-Dimethoxy--6-amino-benzonitrile generated 3,4-dimethoxy-6-Thiocyanate acyl benzonitrile in water and dichloromethane and sulfur anal phosgene (yield is 94% ).It would get thiourea derivative by reacted with 1-(2-furan-carbonyl)-piperazine in ethyl acetate(yield is 90%). Then derived free S-methyl-isothiourea derivatives by methylated with methyl iodide to the reaction with the alkali treatment(yield is 95%). Finally,formamide prazosin was gotten by the reaction of S-methyl isothiourea biological, ammonia, ammonium chloride and urea.
Prazosin Toxicity Data With Reference
| 1. | orl-wmn TDLo:20 µg/kg:BPR,GIT | AIMEAS Annals of Internal Medicine. 97 (1982),455. | ||
| 2. | orl-man TDLo:1143 µg/kg:BPR | AMSVAZ Acta Medica Scandinavica. 213 (1983),157. | ||
| 3. | orl-hmn TDLo:280 µg/kg:CNS,CVS | BMJOAE British Medical Journal. 2 (1976),508. | ||
| 4. | orl-hmn TDLo:1260 µg/kg:CNS,KID | BMJOAE British Medical Journal. 1 (1978),622. |
Prazosin Safety Profile
Human systemic effects by ingestion of very small amounts: somnolence, hallucinations, distorted perceptions, changes in motor activity, decreased blood pressure, nausea or vomiting, and kidney effects. Experimental reproductive effects. When heated to decomposition it emits toxic fumes of NOx.
Prazosin Specification
Prazosin is a sympatholytic drug used to treat high blood pressure (hypertension). It belongs to the class of alpha-adrenergic blockers, which lower blood pressure by relaxing blood vessels. Specifically, prazosin is selective for the alpha-1 receptors on vascular smooth muscle. These receptors are responsible for the vasoconstrictive action of norepinephrine, which would normally raise blood pressure. By blocking these receptors, prazosin reduces blood pressure.
Related Products
- Prazosin
- Prazosin hydrochloride
- 19217-50-6
- 192182-54-0
- 192182-55-1
- 192182-56-2
- 192189-07-4
- 192189-10-9
- 192189-18-7
- 192189-26-7
- 192198-85-9
- 19219-98-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View