-
Name
Raspberry ketone
- EINECS 226-806-4
- CAS No. 5471-51-2
- Article Data75
- CAS DataBase
- Density 1.088 g/cm3
- Solubility Insoluble in water; Soluble in ethanol and diethyl ether
- Melting Point 81-85 °C(lit.)
- Formula C10H12O2
- Boiling Point 292.2 °C at 760 mmHg
- Molecular Weight 164.204
- Flash Point 122.9 °C
- Transport Information
- Appearance white to slightly yellow powder or needles
- Safety 26-36/37/39
- Risk Codes 22
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms 2-Butanone,4-(p-hydroxyphenyl)- (6CI,7CI,8CI);(4-Hydroxybenzyl)acetone;(p-Hydroxybenzyl)acetone;1-(4-Hydroxyphenyl)-3-butanone;1-(p-Hydroxyphenyl)-3-butanone;4-(3-Oxobutyl)phenol;4-(p-Hydroxyphenyl)-2-butanone;Frambinone;NSC 26515;Oxyphenylon;Raspberryketone;Rheosmin;4-(4-Hydroxyphenyl)-2-butanone;
- PSA 37.30000
- LogP 1.91380
Synthetic route
-
-
22214-30-8, 59417-71-9, 3160-35-8
4-(4'-hydroxyphenyl)but-3-en-2-one

-
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen; acetic acid In methanol at 20℃; for 24h; | 99% |
| With 1 wt.% La2O3 supported titania at 200℃; for 3h; Reagent/catalyst; Temperature; Autoclave; | 96% |
| With N-benzylammonium trifluoroacetate; 1,4-dihydro-2,6-dimethyl-3,5-bis<(methylamino)carbonyl>pyridine In tetrahydrofuran at 70℃; for 16h; Sealed tube; | 96% |
-
-
22214-30-8
(E)-4-(4-hydroxyphenyl)-3-buten-2-one

-
A
-
69617-84-1
rac-rhododendrol

-
B
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; nickel(II) chloride hexahydrate; hydrogen In methanol at 0 - 30℃; under 760.051 Torr; Reagent/catalyst; Solvent; Temperature; Time; Reflux; Inert atmosphere; | A 1% B 98% |
| With sodium tetrahydroborate; nickel dichloride In methanol | A 38% B 61% |
| With methanol; formic acid; water; silica gel; palladium dichloride for 12h; Heating; | A 25% B 45% |
| With baker's yeast; ethanol; D-glucose; water at 36℃; for 92h; Product distribution; var. reducing agents, also labelled; | |
| With formic acid; palladium 10% on activated carbon; ammonium formate In methanol at 50℃; for 15h; |

-
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

| Conditions | Yield |
|---|---|
| With acetic acid In tetrahydrofuran; water; acetonitrile at 20℃; for 12h; pH=7.5; | 95% |
| With water; acetic acid; N,N,N',N'-tetramethylguanidine In tetrahydrofuran; acetonitrile at 20℃; | 95% |
-
-
1445875-25-1
4-(4-((triisopropylsilyl)oxy)phenyl)butan-2-one

-
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

| Conditions | Yield |
|---|---|
| With acetic acid In tetrahydrofuran; water; acetonitrile at 20℃; for 12h; pH=7.5; | 95% |
| With water; acetic acid; N,N,N',N'-tetramethylguanidine In tetrahydrofuran; acetonitrile at 20℃; | 75% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 250℃; under 37503.8 Torr; for 3h; Autoclave; Inert atmosphere; Green chemistry; | 94% |
| With hydrogen bromide; acetic acid In water at 130℃; for 4h; Reagent/catalyst; Temperature; Solvent; | 86% |
| With hydrogen bromide; acetic acid |

| Conditions | Yield |
|---|---|
| With 4H2O4S*C18H40N4O12S4 at 40℃; for 8h; Temperature; | 94% |
| With amberlyst-15 In toluene at 20 - 40℃; | 58.1% |
| With 1-butyl-3-methylimidazolium hydrogen sulfate at 20℃; for 24h; Friedel-Crafts Alkylation; regioselective reaction; | 51% |

-
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

| Conditions | Yield |
|---|---|
| In sulfuric acid | 93% |
-
-
74432-58-9
4-(4-(benzyloxy)phenyl)butan-2-one

-
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen In methanol at 18 - 22℃; for 24h; | 93% |
| With 5%-palladium/activated carbon; hydrogen In methanol at 20℃; for 120h; Inert atmosphere; | 93% |

-
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water Industrial scale; | 93% |
-
-
52252-58-1
2-(4-Hydroxybenzyl)acetessigsaeure-ethylester

-
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol at 80℃; for 3h; Solvent; Reagent/catalyst; | 90% |

-
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol; water at 23℃; for 20h; | 87% |
-
-
22214-30-8
(E)-4-(4-hydroxyphenyl)-3-buten-2-one

-
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

| Conditions | Yield |
|---|---|
| With methanol; formic acid; water; silica gel; palladium dichloride for 0.366667h; Irradiation; microwave; | 84% |
| With nickel boride; hydrogen In methanol at 25℃; for 1h; chemoselective reaction; | 75% |
| With baker's yeast; D-glucose; resin XAD 1180 In water at 28℃; for 24h; | 50% |
| With hydrogen |
-
-
59092-94-3
(+)-rhododendrol

-
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

| Conditions | Yield |
|---|---|
| With phosphate buffer; Rhodococcus ruber DSM 44541 cells; acetone at 20℃; for 17h; pH=7.5; | 83% |
| With lyophilised cells of Escherichia coli overexpressing the solvent-tolerant alcohol dehydrogenase from Rhodococcus ruber DSM44541 In aq. buffer at 30℃; for 24h; pH=7.5; Resolution of racemate; Enzymatic reaction; | 73% |

-
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride; zinc(II) chloride In tetrahydrofuran; toluene at 20℃; for 0.75h; Solvent; Temperature; Reagent/catalyst; | 82% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-hydroxy-benzaldehyde; acetone With morpholin-4-ium 2,2,2-trifluoroacetate at 75℃; for 12h; Aldol condensation; Sealed vial; Stage #2: With diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate In tetrahydrofuran at 60℃; for 12h; Inert atmosphere; | 80% |

-
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride; zinc(II) chloride In tetrahydrofuran; toluene at 20℃; for 3h; | 70% |

-
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride; zinc(II) chloride In tetrahydrofuran; toluene at 20℃; for 0.75h; | 66% |

| Conditions | Yield |
|---|---|
| With D218 resin In toluene at 80 - 120℃; for 7h; Temperature; Large scale; | 65% |
| With aluminium trichloride |
-
-
69617-84-1
rac-rhododendrol

-
A
-
59092-94-3, 69617-84-1, 501-96-2
(R)-rhododendrol

-
B
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

| Conditions | Yield |
|---|---|
| With phosphate buffer; Rhodococcus ruber DSM 44541 cells; acetone at 30℃; for 44h; pH=7.5; | A n/a B 44.5% |
| With Thermoanaerobacter ethanolicus secondary alcohol dehydrogenase W110A mutant; NADP In acetone at 50℃; for 24h; pH=8; Enzymatic reaction; enantioselective reaction; | A n/a B n/a |
-
-
590-90-9
1-Hydroxy-3-butanone

-
-
108-95-2
phenol

-
A
-
22409-85-4
4-phenoxybutan-2-one

-
B
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

| Conditions | Yield |
|---|---|
| Zr(4+)-montmorillonite at 100℃; for 48h; | A 0.4% B 28% |
| Zr(4+)-montmorillonite at 100℃; for 48h; Product distribution; further cation-exchanged montmorillonites; also with 3-buten-2-one instead of 4-hydroxybutan-2-one; | A 0.4% B 28% |
| HCl treated montmorillonite at 130℃; under 1500.15 Torr; for 24h; Product distribution / selectivity; |

-
-
22409-85-4
4-phenoxybutan-2-one

-
A
-
16982-89-1
4-methylchroman

-
B
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

| Conditions | Yield |
|---|---|
| With aluminium(III) ion In various solvent(s) at 100℃; for 48h; | A 8% B 10% |
| With aluminium(III) ion In various solvent(s) at 100℃; for 48h; Product distribution; various substrates and catalysts; | A 8% B 10% |
-
-
78-94-4
methyl vinyl ketone

-
-
108-95-2
phenol

-
A
-
22409-85-4
4-phenoxybutan-2-one

-
B
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tert-butyl alcohol at 50℃; for 11h; Product distribution; | |
| Zr(4+)-montmorillonite at 100℃; for 48h; | A 13 % Chromat. B 16 % Chromat. |


-
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

| Conditions | Yield |
|---|---|
| With sodium amalgam; water |

-
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

| Conditions | Yield |
|---|---|
| With aluminium trichloride; benzene |

-
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

| Conditions | Yield |
|---|---|
| With methanol; platinum Hydrogenation; | |
| With diethyl ether; platinum Hydrogenation; | |
| With methanol; palladium on activated charcoal Hydrogenation; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: tetrahydrofuran / Heating 2: H2 / Pd/C / ethyl acetate View Scheme |
-
-
63549-55-3
4-bromophenyl 2,2-dimethylpropanoate

-
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 79 percent / NaHCO3; NaHCO2; tetrabutylammonium bromide / Pd-benzothiazole carbene complex / 8 h / 130 °C 2: 87 percent / NaOH / methanol; H2O / 20 h / 23 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 43 percent / Zr4+-mont / pyridine / 12 h / 100 °C 2: 10 percent / Al3+-mont / various solvent(s) / 48 h / 100 °C / various substrates and catalysts View Scheme |
-
-
598-32-3
2-hydroxy-3-butene

-
-
540-38-5
4-Iodophenol

-
A
-
194279-78-2
C10H12O2

-
B
-
1068658-05-8
C10H12O2

-
C
-
7074-13-7
3-(4'-hydroxyphenyl)butan-2-one

-
D
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

| Conditions | Yield |
|---|---|
| Stage #1: 2-hydroxy-3-butene With dicyclohexylmethylamine; tetrabutylammomium bromide In water at 20℃; for 0.25h; Heck reaction; Stage #2: p-Iodophenol; Kaiser oxime-derived complex on resin base In water at 120℃; for 5h; Heck reaction; Further stages.; | A n/a B n/a C n/a D 81 % Spectr. |

-
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

| Conditions | Yield |
|---|---|
| With potassium hydroxide; water-d2 for 48h; | 100% |
| With potassium hydroxide; water-d2 In 1,4-dioxane at 50℃; for 24h; |
-
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

-
-
69617-84-1
rac-rhododendrol

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol at 20℃; for 2h; Solvent; Enzymatic reaction; | 100% |
| With sodium tetrahydroborate In methanol at 0 - 20℃; for 1.5h; Inert atmosphere; | 95% |
| With sodium tetrahydroborate In methanol; water at 20℃; for 10h; Cooling with ice; | 94% |
-
-
75-77-4
chloro-trimethyl-silane

-
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

-
-
522617-84-1
4-(4-trimethylsilyloxyphenyl)butan-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane | 100% |
| With triethylamine In tetrahydrofuran at 20℃; for 5h; | 91% |
| With triethylamine In tetrahydrofuran at 20℃; |
-
-
13154-24-0
triisopropylsilyl chloride

-
-
5471-51-2
4-(4-hydroxyphenyl)-2-oxobutane

-
-
1445875-25-1
4-(4-((triisopropylsilyl)oxy)phenyl)butan-2-one

| Conditions | Yield |
|---|---|
| With 1H-imidazole In dichloromethane at 0 - 20℃; for 16h; Inert atmosphere; Sealed tube; | 100% |
| With 1H-imidazole at 20℃; | 75% |
| With 1H-imidazole In dichloromethane at 23℃; Inert atmosphere; |
Raspberry ketone Chemical Properties
IUPAC Name: 4-(4-Hydroxyphenyl)butan-2-one
Molecular Formula: C10H12O2
Molecular Weight: 164.2 g/mol
EINECS: 226-806-4
Density: 1.088 g/cm3
Melting Point: 81-85 °C
Boiling Point: 200 °C
Flash Point: 122.9 °C
Appearance: white powder
Index of Refraction: 1.535
Molar Refractivity: 46.97 cm3
Molar Volume: 150.8 cm3
Surface Tension: 41.8 dyne/cm
XLogP3-AA: 1.5
H-Bond Donor: 1
H-Bond Acceptor: 2
Rotatable Bond Count: 3
Tautomer Count: 6
Exact Mass: 164.08373
MonoIsotopic Mass: 164.08373
Topological Polar Surface Area: 37.3
Heavy Atom Count: 12
Canonical SMILES: CC(=O)CCC1=CC=C(C=C1)O
InChI: InChI=1S/C10H12O2/c1-8(11)2-3-9-4-6-10(12)7-5-9/h4-7,12H,2-3H2,1H3
InChIKey: NJGBTKGETPDVIK-UHFFFAOYSA-N
EINECS: 226-806-4
Product Categories: Food and Feed Additive;Aromatic Ketones (substituted)
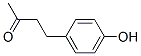
Molecule structure of Raspberry ketone (CAS NO.5471-51-2):
Raspberry ketone Uses
Raspberry ketone (CAS NO.5471-51-2) is the primary aroma compound of red raspberries. It is used in perfumery, in cosmetics, and as a food additive to impart a fruity odor.
Raspberry ketone Toxicity Data With Reference
| 1. | orl-rat LD50:1320 mg/kg | FCTXAV Food and Cosmetics Toxicology. 8 (1970),349. | ||
| 2. | ipr-rat LD50:350 mg/kg | FCTXAV Food and Cosmetics Toxicology. 8 (1970),349. |
Raspberry ketone Consensus Reports
Reported in EPA TSCA Inventory.
Raspberry ketone Safety Profile
Hazard Codes:  Xn,
Xn,  Xi
Xi
Risk Statements: 22
R22:Harmful if swallowed.
Safety Statements: 26-36/37/39
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
WGK Germany: 2
RTECS: EL8925000
Hazard Note: Irritant
Poison by intraperitoneal route. Moderately toxic by ingestion. Flammable liquid. When heated to decomposition it emits acrid smoke and irritating fumes. See also KETONES.
Raspberry ketone Standards and Recommendations
DOT Classification: 3; Label: Flammable Liquid
Raspberry ketone Specification
Raspberry ketone (CAS NO.5471-51-2) is also named as 1-(4-Hydroxyphenyl)-3-butanone ; 1-(p-Hydroxyphenyl)-3-butanone ; 2-Butanone, 4-(4-hydroxyphenyl)- ; 2-Butanone, 4-(p-hydroxyphenyl)- ; 4-(3-Oxobutyl)phenol ; 4-(4-Hydroxyphenyl)-2-butanone ; 4-(p-Hydroxyphenyl)-2-butanone ; 4-(p-Hydroxyphenyl)-2-butanone (natural) ; 4-08-00-00506 (Beilstein Handbook Reference) ; AI3-31812 ; BRN 0776080 ; FEMA No. 2588 ; Frambinone ; Hydroxyphenylbutanone, p- ; NSC 26515 ; Oxyphenalon ; Rasketone ; Rheosmin ; UNII-7QY1MH15BG ; p-Hydroxybenzyl acetone . It is insoluble in water, soluble in ethanol, ether and volatile oil.
Related Products
- Raspberry ketone
- Raspberry ketone glucoside
- 5471-52-3
- 5471-55-6
- 5471-58-9
- 54716-02-8
- 5471-63-6
- 54-71-7
- 547-17-1
- 54717-17-8
- 5471-77-2
- 5471-81-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View