-
Name
Ritonavir
- EINECS 605-001-5
- CAS No. 155213-67-5
- Article Data12
- CAS DataBase
- Density 1.239 g/cm3
- Solubility 5mg/L(ms)
- Melting Point 120-122°C
- Formula C37H48N6O5S2
- Boiling Point 947 °C at 760 mmHg
- Molecular Weight 720.957
- Flash Point 526.6 °C
- Transport Information
- Appearance white powder
- Safety 26-37/39-36/37-39
- Risk Codes 36/38-20/21/22-41
-
Molecular Structure
- Hazard Symbols Xi,Xn
- Synonyms 2,4,7,12-Tetraazatridecan-13-oicacid,10-hydroxy-2-methyl-5-(1-methylethyl)-1-[2-(1-methylethyl)-4-thiazolyl]-3,6-dioxo-8,11-bis(phenylmethyl)-,5-thiazolylmethyl ester, (5S,8S,10S,11S)- (9CI);A 84538;ABT 538;Abbott84538;NSC 693184;Norvir;
- PSA 202.26000
- LogP 7.07790
Synthetic route
-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

-
-
144164-11-4
(2S,3S,5S)-5-Amino-2-(N-((5-thiazolyl)-methoxycarbonyl)amino)-3-hydroxy-1,6-diphenylhexane

-
-
155213-67-5
ritonavir

| Conditions | Yield |
|---|---|
| Stage #1: N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine With N-ethyl-N,N-diisopropylamine; diisopropyl-carbodiimide at 27℃; for 0.5h; Stage #2: (2S,3S,5S)-5-Amino-2-(N-((5-thiazolyl)-methoxycarbonyl)amino)-3-hydroxy-1,6-diphenylhexane for 7h; Temperature; | 91.5% |
| With diethylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In butanone at 41℃; for 0.666667h; Concentration; Temperature; | 87.4% |
| With N-ethyl-N'-(3-diethylaminopropyl)-carbodiimide; 1-hydroxybenzotriazol-hydrate In tetrahydrofuran for 16h; Ambient temperature; Yield given; | |
| Stage #1: N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine With pivaloyl chloride; triethylamine In dichloromethane at 0 - 10℃; Inert atmosphere; Large scale; Stage #2: With dmap In dichloromethane at 0 - 10℃; for 0.5h; Large scale; Stage #3: (2S,3S,5S)-5-Amino-2-(N-((5-thiazolyl)-methoxycarbonyl)amino)-3-hydroxy-1,6-diphenylhexane In dichloromethane at 25 - 35℃; Large scale; |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In ethyl acetate at 50 - 60℃; for 9h; | 85.29% |

| Conditions | Yield |
|---|---|
| Stage #1: C37H53N5O5S With hydrogenchloride In water; ethyl acetate at 0 - 20℃; for 6h; Green chemistry; Stage #2: (5-thiazolyl)methyl 2,2,2-trichloroethanol carbonate With triethylamine In ethyl acetate at 0 - 20℃; for 12h; Green chemistry; | 79% |

-
-
155213-67-5
ritonavir

| Conditions | Yield |
|---|---|
| With PBS In ethanol pH=7.4; Kinetics; Further Variations:; pH-values; |
-
-
6306-52-1
L-valine methylester hydrochloride

-
-
155213-67-5
ritonavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 4-methylmorpholine / CH2Cl2 / Ambient temperature 2: DMAP, Et3N / tetrahydrofuran / 2 h / Heating 3: 0.50 M aq. LiOH / dioxane / 0.5 h / Ambient temperature 4: 1-hydroxybenzotriazole hydrate, N-ethyl-N'-<(dimethylamino)propyl>carbodiimide / tetrahydrofuran / 16 h / Ambient temperature View Scheme |
-
-
144163-97-3
((5-thiazolyl)methyl)-(4-nitrophenyl)carbonate

-
-
155213-67-5
ritonavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 16 percent / tetrahydrofuran / 4 h / Ambient temperature 2: 1-hydroxybenzotriazole hydrate, N-ethyl-N'-<(dimethylamino)propyl>carbodiimide / tetrahydrofuran / 16 h / Ambient temperature View Scheme |
-
-
154212-60-9
N-methyl-N-<(2-isopropyl-4-thiazolyl)methyl>amine

-
-
155213-67-5
ritonavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: DMAP, Et3N / tetrahydrofuran / 2 h / Heating 2: 0.50 M aq. LiOH / dioxane / 0.5 h / Ambient temperature 3: 1-hydroxybenzotriazole hydrate, N-ethyl-N'-<(dimethylamino)propyl>carbodiimide / tetrahydrofuran / 16 h / Ambient temperature View Scheme | |
| Multi-step reaction with 3 steps 1.1: triethylamine / tetrahydrofuran / 6 h / 50 - 60 °C / Green chemistry 2.1: triethylamine; p-toluenesulfonyl chloride / dichloromethane / 3 h / 0 - 10 °C / Green chemistry 2.2: 6 h / 0 - 20 °C / Green chemistry 3.1: hydrogenchloride / ethyl acetate; water / 6 h / 0 - 20 °C / Green chemistry 3.2: 12 h / 0 - 20 °C / Green chemistry View Scheme |
-
-
162537-10-2
N-<<(4-nitrophenyl)oxy>carbonyl>-L-valine methyl ester

-
-
155213-67-5
ritonavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: DMAP, Et3N / tetrahydrofuran / 2 h / Heating 2: 0.50 M aq. LiOH / dioxane / 0.5 h / Ambient temperature 3: 1-hydroxybenzotriazole hydrate, N-ethyl-N'-<(dimethylamino)propyl>carbodiimide / tetrahydrofuran / 16 h / Ambient temperature View Scheme |
-
-
154248-99-4
N-((N-methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)carbonyl)-L-valine methyl ester

-
-
155213-67-5
ritonavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 0.50 M aq. LiOH / dioxane / 0.5 h / Ambient temperature 2: 1-hydroxybenzotriazole hydrate, N-ethyl-N'-<(dimethylamino)propyl>carbodiimide / tetrahydrofuran / 16 h / Ambient temperature View Scheme |

-
-
155213-67-5
ritonavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: H2O / 1 h 2: DMAP, Et3N / tetrahydrofuran / 2 h / Heating 3: 0.50 M aq. LiOH / dioxane / 0.5 h / Ambient temperature 4: 1-hydroxybenzotriazole hydrate, N-ethyl-N'-<(dimethylamino)propyl>carbodiimide / tetrahydrofuran / 16 h / Ambient temperature View Scheme |
-
-
224631-15-6
N-[[N-methyl-N-[(2-isopropyl-4-thiazolyl)methyl]amino]carbonyl]-L-valinyl hydroxysuccinimide ester

-
-
144164-11-4
(2S,3S,5S)-5-Amino-2-(N-((5-thiazolyl)-methoxycarbonyl)amino)-3-hydroxy-1,6-diphenylhexane

-
-
155213-67-5
ritonavir

| Conditions | Yield |
|---|---|
| In ethyl acetate at 0 - 20℃; for 18h; | |
| In ethyl acetate at 0 - 20℃; for 18h; |

-
-
144164-11-4
(2S,3S,5S)-5-Amino-2-(N-((5-thiazolyl)-methoxycarbonyl)amino)-3-hydroxy-1,6-diphenylhexane

-
-
155213-67-5
ritonavir

| Conditions | Yield |
|---|---|
| In ethyl acetate Solvent; | 55.7 g |
-
-
162849-95-8
(2S,3S,5S)-5-(t-butyloxycarbonylamino)-2-(N-((5-thiazolyl)methoxycarbonyl)amino)-3-hydroxy-1,6-diphenylhexane

-
-
155213-67-5
ritonavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydrogenchloride / water; ethyl acetate / 3 h / 50 °C 2: ethyl acetate View Scheme |

-
-
155213-67-5
ritonavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: dichloromethane / 1 h / 10 °C 2: hydrogenchloride / ethyl acetate; water / 4 h / 10 - 20 °C 3: sodium hydrogencarbonate / ethyl acetate / 9 h / 50 - 60 °C View Scheme |
-
-
154212-61-0
N-((N-Methyl-N-((2-isopropyl-4-thiazolyl)methyl)amino)-carbonyl)-L-valine

-
-
155213-67-5
ritonavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: thionyl chloride / dichloromethane; N,N-dimethyl-formamide / 1 h / 0 - 10 °C 2: dichloromethane / 1 h / 10 °C 3: hydrogenchloride / ethyl acetate; water / 4 h / 10 - 20 °C 4: sodium hydrogencarbonate / ethyl acetate / 9 h / 50 - 60 °C View Scheme |
-
-
78221-33-7
(S)-3-methyl-2-[(2,2,2-trichloroethoxy)carbonylamino]butyric acid

-
-
155213-67-5
ritonavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: triethylamine / tetrahydrofuran / 6 h / 50 - 60 °C / Green chemistry 2.1: triethylamine; p-toluenesulfonyl chloride / dichloromethane / 3 h / 0 - 10 °C / Green chemistry 2.2: 6 h / 0 - 20 °C / Green chemistry 3.1: hydrogenchloride / ethyl acetate; water / 6 h / 0 - 20 °C / Green chemistry 3.2: 12 h / 0 - 20 °C / Green chemistry View Scheme |
-
-
75-18-3
dimethylsulfide

-
-
155213-67-5
ritonavir

-
-
875435-42-0
N1-((1S,3S,4S)-1-benzyl-3-[(methylthio)methoxy]-5-phenyl-4-{[(1,3-thiazol-5-ylmethoxy)carbonyl]amino}pentyl)-N2-{[[(2-isopropyl-1,3-thiazol-4-yl)methyl](methyl)amino]carbonyl}-L-valinamide

| Conditions | Yield |
|---|---|
| With dibenzoyl peroxide In acetonitrile at 0 - 20℃; for 2.33333h; | 84% |
| With dibenzoyl peroxide In acetonitrile at 0 - 20℃; for 2.33333h; | 84% |
-
-
875435-89-5
4-((di-tert-butoxyphosphoryl)oxy)-3,3-dimethylbutanoic acid

-
-
155213-67-5
ritonavir

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 86h; | 81% |
-
-
67746-43-4
dibenzyl N,N-diethylphosphoramidite

-
-
155213-67-5
ritonavir

-
-
875435-73-7
N1-((1S,3S,4S)-1-benzyl-3-{[bis(benzyloxy)phosphoryl]oxy}-5-phenyl-4-{[(1,3-thiazol-5-ylmethoxy)carbonyl]amino}pentyl)-N2-{[[(2-isopropyl-1,3-thiazol-4-yl)methyl](methyl)amino]carbonyl}-L-valinamide

| Conditions | Yield |
|---|---|
| Stage #1: dibenzyl N,N-diethylphosphoramidite; ritonavir With 1H-tetrazole In tetrahydrofuran at 20℃; for 4h; Stage #2: With 3-chloro-benzenecarboperoxoic acid In tetrahydrofuran; Dichlorodifluoromethane at -45 - 20℃; for 1h; | 76% |
-
-
352-93-2
diethyl sulphide

-
-
155213-67-5
ritonavir

-
-
875435-55-5
N1-((1S,3S,4S)-1-benzyl-3-[1-(ethylthio)ethoxy]-5-phenyl-4-{[(1,3-thiazol-5-ylmethoxy)carbonyl]amino}pentyl)-N2-{[[(2-isopropyl-1,3-thiazol-4-yl)methyl](methyl)amino]carbonyl}-L-valinamide

| Conditions | Yield |
|---|---|
| With dibenzoyl peroxide In acetonitrile at 0 - 20℃; for 2.33333h; | 75% |
| With dibenzoyl peroxide In acetonitrile at 0℃; for 3h; | 75% |
-
-
592-65-4
diisobutyl sulfide

-
-
155213-67-5
ritonavir

-
-
875436-09-2
N1-((1S,3S,4S)-1-benzyl-3-[1-(isobutylthio)-2-methylpropoxy]-5-phenyl-4-{[(1,3-thiazol-5-ylmethoxy)carbonyl]amino}pentyl)-N2-{[[(2-isopropyl-1,3-thiazol-4-yl)methyl](methyl)amino]carbonyl}-L-valinamide

| Conditions | Yield |
|---|---|
| With dibenzoyl peroxide In acetonitrile at 0 - 20℃; for 2.33333h; | 75% |
| With dibenzoyl peroxide In acetonitrile at 0℃; for 1.5h; | 75% |

| Conditions | Yield |
|---|---|
| With dmap; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 16h; | 72% |

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 5h; | 70.3% |
-
-
544-40-1
Dibutyl sulfide

-
-
155213-67-5
ritonavir

-
-
875436-05-8
N1-((1S,3S,4S)-1-benzyl-3-[1-(butylthio)butoxy]-5-phenyl-4-{[(1,3-thiazol-5-ylmethoxy)carbonyl]amino}pentyl)-N2-{[[(2-isopropyl-1,3-thiazol-4-yl)methyl](methyl)amino]carbonyl}-L-valinamide

| Conditions | Yield |
|---|---|
| With dibenzoyl peroxide In acetonitrile at 0 - 20℃; for 2.33333h; | 68% |
| With dibenzoyl peroxide In acetonitrile at 0 - 20℃; for 7h; | 68% |

| Conditions | Yield |
|---|---|
| In 1,2-dichloro-ethane at 75℃; for 6h; |
-
-
155213-67-5
ritonavir

| Conditions | Yield |
|---|---|
| With Dess-Martin periodane In dichloromethane at 20℃; for 6h; | |
| With Dess-Martin periodane |

| Conditions | Yield |
|---|---|
| With 1H-tetrazole In tetrahydrofuran at 20℃; for 4h; |

| Conditions | Yield |
|---|---|
| With human CYP3A4; NADPH; NADPH-cytochrome P450 reductase In aq. phosphate buffer at 37℃; for 0.333333h; pH=7.7; Enzymatic reaction; | |
| With cytochrome P450 monooxygenases from Actinosynnema mirum at 25℃; for 24h; pH=7.5; Reagent/catalyst; Enzymatic reaction; |


| Conditions | Yield |
|---|---|
| With human CYP2B6; NADPH; NADPH-cytochrome P450 reductase In aq. phosphate buffer at 37℃; for 0.333333h; pH=7.7; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With human CYP3A4; NADPH; NADPH-cytochrome P450 reductase In aq. phosphate buffer at 37℃; for 0.333333h; pH=7.7; Enzymatic reaction; |
-
-
155213-67-5
ritonavir

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethyl acetate Concentration; Temperature; Solvent; Inert atmosphere; |
-
-
155213-67-5
ritonavir

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 75 °C 2: 2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride / 115 °C View Scheme |

| Conditions | Yield |
|---|---|
| at 75℃; |
Ritonavir Chemical Properties
Molecular structure of Ritonavir (CAS NO.155213-67-5) is:
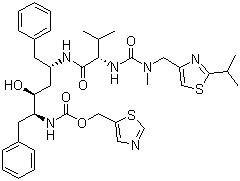
Product Name: Ritonavir
CAS Registry Number: 155213-67-5
IUPAC Name: 1,3-thiazol-5-ylmethylN-[(2S,3S,5S)-3-hydroxy-5-[[(2S)-3-methyl-2-[[methyl-[(2-propan-2-yl-1,3-thiazol-4-yl)methyl]carbamoyl]amino]butanoyl]amino]-1,66-diphenylhexan-2-yl]carbamate
Molecular Weight: 720.94422 [g/mol]
Molecular Formula: C37H48N6O5S2
XLogP3-AA: 6
H-Bond Donor: 4
H-Bond Acceptor: 7
Surface Tension: 53.7 dyne/cm
Density: 1.239 g/cm3
Flash Point: 526.6 °C
Enthalpy of Vaporization: 144.39 kJ/mol
Boiling Point: 947 °C at 760 mmHg
Classification Code: Anti-HIV Agents; Anti-Infective Agents; Anti-Retroviral Agents; Antiretroviral; Antiviral; Antiviral Agents; Enzyme Inhibitors; HIV Protease Inhibitors; Protease Inhibitors; Protease inhibitor
Ritonavir History
Ritonavir (CAS NO.155213-67-5) is manufactured as Norvir by Abbott Laboratories. Research that led to the drug's development was financed by a $3,500,000 federal grant through the National Institutes of Health (NIH) and over $200,000,000 by Abbott Labs. Most of the $200,000,000 figure cited by Abbott paid for clinical trials-despite NIH offering to pay for them-because Abbott was concerned about "public interest" responses to the high prices they projected Norvir would command.The FDA approved ritonavir on March 1, 1996, making it the seventh approved antiretroviral drug in the United States. In 2003, Abbott raised the price of a Norvir course from USD $1.71 per day to $8.57 per day, leading to claims of price gouging by patients' groups and some members of Congress. Consumer group Essential Inventions petitioned the NIH to override the Norvir patent, but the NIH announced on August 4, 2004 that it would not invoke its legal right to allow generic production of Norvir, citing potential adverse effects on the pharmaceutical market.
Ritonavir Uses
Ritonavir (CAS NO.155213-67-5) is an antiretroviral drug from the protease inhibitor class used to treat HIV infection and AIDS.
Ritonavir Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD | oral | > 2500mg/kg (2500mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: ATAXIA LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 40, Pg. 2683, 1998. |
| mouse | LDLo | intravenous | 65mg/kg (65mg/kg) | BEHAVIORAL: TREMOR BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: ATAXIA | Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 40, Pg. 2683, 1998. |
| rat | LD | oral | > 2500mg/kg (2500mg/kg) | BEHAVIORAL: TREMOR BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 40, Pg. 2683, 1998. |
| rat | LDLo | intravenous | 35mg/kg (35mg/kg) | BEHAVIORAL: TREMOR BEHAVIORAL: ATAXIA LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 40, Pg. 2683, 1998. |
Ritonavir Specification
Ritonavir , its cas register number is 155213-67-5. It also can be called Ritonavir [USAN] ; 5-Thiazolylmethyl ((alphaS)-alpha-((1S,3S)-1-hydroxy-3-((2S)-2-(3-((2-isopropyl-4-thiazolyl)methyl)-3-methylureido)-3-methylbutyramido)-4-phenylbutyl)phenethyl)carbamate ; 5-Thiazolylmethyl ((alphaS)-alpha-((1S,3S-1-hydroxy-3-((2S)-2-(3-((2-isopropyl-4-thiazolyl)methyl)-3-methylureido)-3-methylbutyramido)-4-phenylbutyl)phenethyl)carbamate ; 2,4,7,12-Tetraazatridecan-13-oic acid, 10-hydroxy-2-methyl-5-(1-methylethyl)-1-(2-(1-methylethyl)-4-thiazolyl)-3,6-dioxo-8,11-bis(phenylmethyl)-, 5-thiazolylmethyl ester, (5S-(5R*,8R*,10R*,11R*))- .It is a white powder.
Related Products
- Ritonavir
- 15521-65-0
- 15522-69-7
- 15522-71-1
- 15522-73-3
- 15523-24-7
- 1552-42-7
- 15525-15-2
- 155266-68-5
- 155268-88-5
- 155270-53-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View