-
Name
Seratrodast
- EINECS 692-169-8
- CAS No. 112665-43-7
- Article Data8
- CAS DataBase
- Density 1.14 g/cm3
- Solubility
- Melting Point 118-120 °C
- Formula C22H26O4
- Boiling Point 522.3 °C at 760 mmHg
- Molecular Weight 354.446
- Flash Point 283.8 °C
- Transport Information
- Appearance pale orange solid
- Safety
- Risk Codes R36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Benzeneheptanoicacid, z-(2,4,5-trimethyl-3,6-dioxo-1,4-cyclohexadien-1-yl)-,(?à)-;7-(3,5,6-Trimethyl-1,4-benzoquinon-2-yl)-7-phenylheptanoic acid;A 73001;AA 2414;ABT 001;Abbott 73001;Bronica;
- PSA 71.44000
- LogP 4.61000
Synthetic route

| Conditions | Yield |
|---|---|
| Stage #1: 1,4-dimethoxy-2,3,5-trimethyl-benzene; C13H19NO2 With sodium nitrate; aluminum isopropoxide at 47℃; for 2h; Stage #2: With dipotassium peroxodisulfate; sodium sulfate at 55℃; for 0.833333h; Stage #3: With niobium(V) oxide for 3.6h; Temperature; | 99.2% |

-
-
148989-82-6
7-(2-hydroxy-3,4,6-trimethylphenyl)-7-phenylheptanoic acid

-
-
112665-43-7
seratrodast

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In water; acetonitrile | 94.1% |
-
-
700-13-0
Trimethylhydroquinone

-
-
109-63-7
boron trifluoride diethyl etherate

-
-
103187-18-4
7-hydroxy-7-phenylheptanoic acid

-
-
112665-43-7
seratrodast

| Conditions | Yield |
|---|---|
| With iron(III) chloride In tetrahydrofuran; water; toluene | 73% |


| Conditions | Yield |
|---|---|
| Stage #1: 2,3,5-Trimethyl-1,4-benzoquinone; phenyl-2-oxocanone With diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate at 20℃; for 0.5h; Stage #2: With hafnium tetrakis(trifluoromethanesulfonate) at 40℃; for 6h; | 46% |
-
-
148989-73-5
7-(2,5-Dihydroxy-3,4,6-trimethyl-phenyl)-7-phenyl-heptanoic acid

-
-
112665-43-7
seratrodast

| Conditions | Yield |
|---|---|
| With iron(III) chloride In tetrahydrofuran for 0.5h; Ambient temperature; Yield given; | |
| With iron(III) chloride In tetrahydrofuran at 25℃; for 0.5h; Inert atmosphere; |
-
-
14794-32-2
ethyl 6-(chloroformyl)hexanoate

-
-
112665-43-7
seratrodast

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: AlCl3 / 2 h / 90 °C 2: NaBH4 / methanol / 0.5 h / 0 °C 3: 95 percent / 2.5 N NaOH / tetrahydrofuran / 3 h / 70 °C 4: boron trifluoride etherate / toluene / 2 h / 60 °C 5: aq. FeCl3 / tetrahydrofuran / 0.5 h / Ambient temperature View Scheme |
-
-
33018-91-6
7-ethoxy-7-oxoheptanoic acid

-
-
112665-43-7
seratrodast

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 98 percent / SOCl2 / CH2Cl2 / 2 h / 50 °C 2: AlCl3 / 2 h / 90 °C 3: NaBH4 / methanol / 0.5 h / 0 °C 4: 95 percent / 2.5 N NaOH / tetrahydrofuran / 3 h / 70 °C 5: boron trifluoride etherate / toluene / 2 h / 60 °C 6: aq. FeCl3 / tetrahydrofuran / 0.5 h / Ambient temperature View Scheme |
-
-
112665-41-5
ethyl 7-oxo-7-phenylheptanoate

-
-
112665-43-7
seratrodast

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: NaBH4 / methanol / 0.5 h / 0 °C 2: 95 percent / 2.5 N NaOH / tetrahydrofuran / 3 h / 70 °C 3: boron trifluoride etherate / toluene / 2 h / 60 °C 4: aq. FeCl3 / tetrahydrofuran / 0.5 h / Ambient temperature View Scheme |
-
-
112665-42-6
7-hydroxy-7-phenyl-heptanoic acid ethyl ester

-
-
112665-43-7
seratrodast

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 95 percent / 2.5 N NaOH / tetrahydrofuran / 3 h / 70 °C 2: boron trifluoride etherate / toluene / 2 h / 60 °C 3: aq. FeCl3 / tetrahydrofuran / 0.5 h / Ambient temperature View Scheme |
-
-
103187-18-4
7-hydroxy-7-phenylheptanoic acid

-
-
112665-43-7
seratrodast

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: boron trifluoride etherate / toluene / 2 h / 60 °C 2: aq. FeCl3 / tetrahydrofuran / 0.5 h / Ambient temperature View Scheme |
-
-
108-86-1
bromobenzene

-
-
112665-43-7
seratrodast

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: magnesium; iodine / tetrahydrofuran / 1 h / Sealed tube; Inert atmosphere; Reflux 1.2: 20 °C / Sealed tube; Inert atmosphere 1.3: Sealed tube; Inert atmosphere; Reflux; Dean-Stark 2.1: borane-THF / tetrahydrofuran / 2.5 h / 20 °C / Sealed tube; Inert atmosphere 2.2: 4 h / 20 °C / Sealed tube; Inert atmosphere 3.1: pyridinium chlorochromate / dichloromethane / 24 h / 0 - 20 °C 4.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 16 h / 35 °C / Inert atmosphere; Sealed tube 5.1: diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate / 0.5 h / 20 °C 5.2: 6 h / 40 °C View Scheme |
-
-
502-42-1
cycloheptanone

-
-
112665-43-7
seratrodast

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: magnesium; iodine / tetrahydrofuran / 1 h / Sealed tube; Inert atmosphere; Reflux 1.2: 20 °C / Sealed tube; Inert atmosphere 1.3: Sealed tube; Inert atmosphere; Reflux; Dean-Stark 2.1: borane-THF / tetrahydrofuran / 2.5 h / 20 °C / Sealed tube; Inert atmosphere 2.2: 4 h / 20 °C / Sealed tube; Inert atmosphere 3.1: pyridinium chlorochromate / dichloromethane / 24 h / 0 - 20 °C 4.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 16 h / 35 °C / Inert atmosphere; Sealed tube 5.1: diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate / 0.5 h / 20 °C 5.2: 6 h / 40 °C View Scheme |
-
-
25308-75-2, 71340-35-7, 152016-48-3
1-Phenylcycloheptene

-
-
112665-43-7
seratrodast

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: borane-THF / tetrahydrofuran / 2.5 h / 20 °C / Sealed tube; Inert atmosphere 1.2: 4 h / 20 °C / Sealed tube; Inert atmosphere 2.1: pyridinium chlorochromate / dichloromethane / 24 h / 0 - 20 °C 3.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 16 h / 35 °C / Inert atmosphere; Sealed tube 4.1: diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate / 0.5 h / 20 °C 4.2: 6 h / 40 °C View Scheme |
-
-
92035-59-1
2-phenylcycloheptan-1-ol

-
-
112665-43-7
seratrodast

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: pyridinium chlorochromate / dichloromethane / 24 h / 0 - 20 °C 2.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 16 h / 35 °C / Inert atmosphere; Sealed tube 3.1: diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate / 0.5 h / 20 °C 3.2: 6 h / 40 °C View Scheme |
-
-
14996-78-2
2-phenylcycloheptanone

-
-
112665-43-7
seratrodast

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 16 h / 35 °C / Inert atmosphere; Sealed tube 2.1: diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate / 0.5 h / 20 °C 2.2: 6 h / 40 °C View Scheme |

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane; N,N-dimethyl-formamide at 20℃; Cooling with ice; | 100% |
-
-
67-56-1
methanol

-
-
112665-43-7
seratrodast

| Conditions | Yield |
|---|---|
| With sulfuric acid at 80℃; for 2h; | 98% |
-
-
100-02-7
4-nitro-phenol

-
-
112665-43-7
seratrodast

-
-
112665-40-4
p-nitrophenyl 7-(3,5,6-trimethyl-1,4-benzoquinon-2-yl)-7-phenylheptanoate

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In dichloromethane 1) 0 deg C, 30 min, 2) room temperature, 1 h; | 91% |
-
-
112665-43-7
seratrodast

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 91 percent / dicyclohexyl carbodiimide / CH2Cl2 / 1) 0 deg C, 30 min, 2) room temperature, 1 h 2: 87 percent / aq. conc. ammonia / tetrahydrofuran / 4 h / Ambient temperature View Scheme |
-
-
112665-43-7
seratrodast

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 91 percent / dicyclohexyl carbodiimide / CH2Cl2 / 1) 0 deg C, 30 min, 2) room temperature, 1 h 2: 81 percent / tetrahydrofuran / Ambient temperature View Scheme |
-
-
112665-43-7
seratrodast

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 91 percent / dicyclohexyl carbodiimide / CH2Cl2 / 1) 0 deg C, 30 min, 2) room temperature, 1 h View Scheme |
-
-
112665-43-7
seratrodast

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 91 percent / dicyclohexyl carbodiimide / CH2Cl2 / 1) 0 deg C, 30 min, 2) room temperature, 1 h 2: 85 percent / tetrahydrofuran / Ambient temperature View Scheme |
-
-
2221-00-3
4-(N-imidazolyl)aniline

-
-
112665-43-7
seratrodast

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; triethylamine; 1-ethyl-3-(3-dimethylamino-propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 20h; |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
112665-43-7
seratrodast
-
-
112665-43-7
seratrodast

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: dmap / dichloromethane; N,N-dimethyl-formamide / 20 °C / Cooling with ice 2: hydrogenchloride / dichloromethane; ethyl acetate / 2 h / 20 °C / Cooling with ice View Scheme |
Seratrodast Chemical Properties
Molecular structure of Seratrodast (CAS NO.112665-43-7) is:
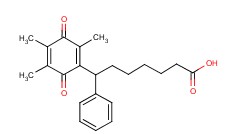
Product Name: Seratrodast
CAS Registry Number: 112665-43-7
IUPAC Name: 7-phenyl-7-(2,4,5-trimethyl-3,6-dioxocyclohexa-1,4-dien-1-yl)heptanoic acid
Molecular Weight: 354.43944 [g/mol]
Molecular Formula: C22H26O4
XLogP3-AA: 4.4
H-Bond Donor: 1
H-Bond Acceptor: 4
Melting point: 118-120 °C
Density: 1.14 g/cm3
Flash Point: 283.8 °C
Enthalpy of Vaporization: 83.77 kJ/mol
Boiling Point: 522.3 °C at 760 mmHg
Vapour Pressure: 9.74E-12 mmHg at 25°C
Surface Tension: 45.2 dyne/cm
Other Registry Number: 103186-19-2
Product Categories: API;Heterocyclic Compounds;Intermediates & Fine Chemicals;Pharmaceuticals
Seratrodast Uses
Seratrodast (CAS NO.112665-43-7) is a thromboxane receptor antagonist used primarily in the treatment of asthma.
Seratrodast Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| monkey | LD | oral | > 2gm/kg (2000mg/kg) | GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 38, Pg. 394, 1996. |
| mouse | LD50 | intraperitoneal | 1100mg/kg (1100mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 26, Pg. 953, 1995. | |
| mouse | LD50 | oral | 1520mg/kg (1520mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 26, Pg. 953, 1995. | |
| mouse | LD50 | subcutaneous | > 5gm/kg (5000mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 26, Pg. 953, 1995. | |
| rat | LD50 | intraperitoneal | 1350mg/kg (1350mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 26, Pg. 953, 1995. | |
| rat | LD50 | oral | 3750mg/kg (3750mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 26, Pg. 953, 1995. | |
| rat | LD50 | subcutaneous | > 5gm/kg (5000mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 26, Pg. 953, 1995. |
Seratrodast Specification
Seratrodast , its cas register number is 112665-43-7. It also can be called (+-)-7-(3,5,6-Trimethyl-1,4-benzoquinon-2-yl)-7-phenylheptanoic acid ; (+-)-2,4,5-Trimethyl-3,6-dioxo-zeta-phenyl-1,4-cyclohexadiene-1-heptanoic acid ; Benzeneheptanoic acid, zeta-(2,4,5-trimethyl-3,6-dioxo-1,4-cyclohexadien-1-yl)-, (+-)- .It is a pale orange solid.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View