-
Name
Silodosin
- EINECS
- CAS No. 160970-64-9
- Article Data1
- CAS DataBase
- Density 1.249 g/cm3
- Solubility
- Melting Point
- Formula C25H32F3N3O4
- Boiling Point 601.4 °C at 760 mmHg
- Molecular Weight 495.542
- Flash Point 317.5 °C
- Transport Information
- Appearance colorless to pink solid
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 1H-Indole-7-carboxamide,2,3-dihydro-1-(3-hydroxypropyl)-5-[2-[[2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl]amino]propyl]-;2,3-Dihydro-1-(3-hydroxypropyl)-5-[2-[[2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethyl]amino]propyl]-1H-indole-7-carboxamide;
- PSA 97.05000
- LogP 4.22730
Synthetic route

-
-
160970-64-9
1-(3-hydroxypropyl)-5-(2-((2-(2-(2,2,2-trifluoroethoxy)phenoxy)ethyl)amino)propyl)indoline-7-carboxamide

| Conditions | Yield |
|---|---|
| With water; dihydrogen peroxide; sodium hydroxide In dimethyl sulfoxide at 0 - 25℃; | 87% |

-
-
160970-64-9
1-(3-hydroxypropyl)-5-(2-((2-(2-(2,2,2-trifluoroethoxy)phenoxy)ethyl)amino)propyl)indoline-7-carboxamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: acetic anhydride / tetrahydrofuran / 24 h / 70 °C 2: hydrogen; palladium 10% on activated carbon / methanol; tetrahydrofuran / 48 h / 40 °C 3: sodium tris(acetoxy)borohydride / 1,2-dichloro-ethane / 12 h / 20 °C 4: sodium hydroxide; water / methanol / 2 h / 20 °C 5: sodium hydroxide; water; dihydrogen peroxide / dimethyl sulfoxide / 0 - 25 °C View Scheme |

-
-
160970-64-9
1-(3-hydroxypropyl)-5-(2-((2-(2-(2,2,2-trifluoroethoxy)phenoxy)ethyl)amino)propyl)indoline-7-carboxamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1.1: triethylamine / dichloromethane / 8 h / 0 - 25 °C / Inert atmosphere 2.1: trichlorophosphate / 1,2-dichloro-ethane / 1 h / 0 °C / Inert atmosphere 2.2: 2 h / 0 - 80 °C / Inert atmosphere 3.1: ammonium acetate; acetic acid / 4 h / 80 °C 4.1: sodium tetrahydroborate / dichloromethane; methanol / 1 h / 0 - 25 °C 5.1: trichlorophosphate / 1 h / 0 °C / Inert atmosphere 5.2: 2 h / 0 - 80 °C / Inert atmosphere 6.1: hydroxylamine hydrochloride; pyridine / tetrahydrofuran / 12 h / 70 °C 7.1: acetic anhydride / tetrahydrofuran / 24 h / 70 °C 8.1: hydrogen; palladium 10% on activated carbon / methanol; tetrahydrofuran / 48 h / 40 °C 9.1: sodium tris(acetoxy)borohydride / 1,2-dichloro-ethane / 12 h / 20 °C 10.1: sodium hydroxide; water / methanol / 2 h / 20 °C 11.1: sodium hydroxide; water; dihydrogen peroxide / dimethyl sulfoxide / 0 - 25 °C View Scheme |

-
-
160970-64-9
1-(3-hydroxypropyl)-5-(2-((2-(2-(2,2,2-trifluoroethoxy)phenoxy)ethyl)amino)propyl)indoline-7-carboxamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: trichlorophosphate / 1,2-dichloro-ethane / 1 h / 0 °C / Inert atmosphere 1.2: 2 h / 0 - 80 °C / Inert atmosphere 2.1: ammonium acetate; acetic acid / 4 h / 80 °C 3.1: sodium tetrahydroborate / dichloromethane; methanol / 1 h / 0 - 25 °C 4.1: trichlorophosphate / 1 h / 0 °C / Inert atmosphere 4.2: 2 h / 0 - 80 °C / Inert atmosphere 5.1: hydroxylamine hydrochloride; pyridine / tetrahydrofuran / 12 h / 70 °C 6.1: acetic anhydride / tetrahydrofuran / 24 h / 70 °C 7.1: hydrogen; palladium 10% on activated carbon / methanol; tetrahydrofuran / 48 h / 40 °C 8.1: sodium tris(acetoxy)borohydride / 1,2-dichloro-ethane / 12 h / 20 °C 9.1: sodium hydroxide; water / methanol / 2 h / 20 °C 10.1: sodium hydroxide; water; dihydrogen peroxide / dimethyl sulfoxide / 0 - 25 °C View Scheme |
-
-
350797-52-3
1-(3-benzoyloxypropyl)-5-formyl-2,3-dihydroindole

-
-
160970-64-9
1-(3-hydroxypropyl)-5-(2-((2-(2-(2,2,2-trifluoroethoxy)phenoxy)ethyl)amino)propyl)indoline-7-carboxamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: ammonium acetate; acetic acid / 4 h / 80 °C 2.1: sodium tetrahydroborate / dichloromethane; methanol / 1 h / 0 - 25 °C 3.1: trichlorophosphate / 1 h / 0 °C / Inert atmosphere 3.2: 2 h / 0 - 80 °C / Inert atmosphere 4.1: hydroxylamine hydrochloride; pyridine / tetrahydrofuran / 12 h / 70 °C 5.1: acetic anhydride / tetrahydrofuran / 24 h / 70 °C 6.1: hydrogen; palladium 10% on activated carbon / methanol; tetrahydrofuran / 48 h / 40 °C 7.1: sodium tris(acetoxy)borohydride / 1,2-dichloro-ethane / 12 h / 20 °C 8.1: sodium hydroxide; water / methanol / 2 h / 20 °C 9.1: sodium hydroxide; water; dihydrogen peroxide / dimethyl sulfoxide / 0 - 25 °C View Scheme |
-
-
350797-53-4
1-(3-benzoyloxypropyl)-5-(2-nitro-1-propenyl)-2,3-dihydroindole

-
-
160970-64-9
1-(3-hydroxypropyl)-5-(2-((2-(2-(2,2,2-trifluoroethoxy)phenoxy)ethyl)amino)propyl)indoline-7-carboxamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: sodium tetrahydroborate / dichloromethane; methanol / 1 h / 0 - 25 °C 2.1: trichlorophosphate / 1 h / 0 °C / Inert atmosphere 2.2: 2 h / 0 - 80 °C / Inert atmosphere 3.1: hydroxylamine hydrochloride; pyridine / tetrahydrofuran / 12 h / 70 °C 4.1: acetic anhydride / tetrahydrofuran / 24 h / 70 °C 5.1: hydrogen; palladium 10% on activated carbon / methanol; tetrahydrofuran / 48 h / 40 °C 6.1: sodium tris(acetoxy)borohydride / 1,2-dichloro-ethane / 12 h / 20 °C 7.1: sodium hydroxide; water / methanol / 2 h / 20 °C 8.1: sodium hydroxide; water; dihydrogen peroxide / dimethyl sulfoxide / 0 - 25 °C View Scheme |
-
-
496-15-1
1-indoline

-
-
160970-64-9
1-(3-hydroxypropyl)-5-(2-((2-(2-(2,2,2-trifluoroethoxy)phenoxy)ethyl)amino)propyl)indoline-7-carboxamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1.1: potassium carbonate / acetonitrile / 12 h / 90 °C 2.1: triethylamine / dichloromethane / 8 h / 0 - 25 °C / Inert atmosphere 3.1: trichlorophosphate / 1,2-dichloro-ethane / 1 h / 0 °C / Inert atmosphere 3.2: 2 h / 0 - 80 °C / Inert atmosphere 4.1: ammonium acetate; acetic acid / 4 h / 80 °C 5.1: sodium tetrahydroborate / dichloromethane; methanol / 1 h / 0 - 25 °C 6.1: trichlorophosphate / 1 h / 0 °C / Inert atmosphere 6.2: 2 h / 0 - 80 °C / Inert atmosphere 7.1: hydroxylamine hydrochloride; pyridine / tetrahydrofuran / 12 h / 70 °C 8.1: acetic anhydride / tetrahydrofuran / 24 h / 70 °C 9.1: hydrogen; palladium 10% on activated carbon / methanol; tetrahydrofuran / 48 h / 40 °C 10.1: sodium tris(acetoxy)borohydride / 1,2-dichloro-ethane / 12 h / 20 °C 11.1: sodium hydroxide; water / methanol / 2 h / 20 °C 12.1: sodium hydroxide; water; dihydrogen peroxide / dimethyl sulfoxide / 0 - 25 °C View Scheme |
-
-
350797-54-5
1-(3-benzoyloxypropyl)-5-(2-nitropropyl)-2,3-dihydroindole

-
-
160970-64-9
1-(3-hydroxypropyl)-5-(2-((2-(2-(2,2,2-trifluoroethoxy)phenoxy)ethyl)amino)propyl)indoline-7-carboxamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: trichlorophosphate / 1 h / 0 °C / Inert atmosphere 1.2: 2 h / 0 - 80 °C / Inert atmosphere 2.1: hydroxylamine hydrochloride; pyridine / tetrahydrofuran / 12 h / 70 °C 3.1: acetic anhydride / tetrahydrofuran / 24 h / 70 °C 4.1: hydrogen; palladium 10% on activated carbon / methanol; tetrahydrofuran / 48 h / 40 °C 5.1: sodium tris(acetoxy)borohydride / 1,2-dichloro-ethane / 12 h / 20 °C 6.1: sodium hydroxide; water / methanol / 2 h / 20 °C 7.1: sodium hydroxide; water; dihydrogen peroxide / dimethyl sulfoxide / 0 - 25 °C View Scheme |
-
-
350797-55-6
1-(3-benzoyloxypropyl)-7-formyl-5-(2-nitropropyl)-2,3-dihydroindole

-
-
160970-64-9
1-(3-hydroxypropyl)-5-(2-((2-(2-(2,2,2-trifluoroethoxy)phenoxy)ethyl)amino)propyl)indoline-7-carboxamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: hydroxylamine hydrochloride; pyridine / tetrahydrofuran / 12 h / 70 °C 2: acetic anhydride / tetrahydrofuran / 24 h / 70 °C 3: hydrogen; palladium 10% on activated carbon / methanol; tetrahydrofuran / 48 h / 40 °C 4: sodium tris(acetoxy)borohydride / 1,2-dichloro-ethane / 12 h / 20 °C 5: sodium hydroxide; water / methanol / 2 h / 20 °C 6: sodium hydroxide; water; dihydrogen peroxide / dimethyl sulfoxide / 0 - 25 °C View Scheme |
-
-
350797-56-7
1-(3-benzoyloxypropyl)-7-cyano-5-(2-nitropropyl)-2,3-dihydroindole

-
-
160970-64-9
1-(3-hydroxypropyl)-5-(2-((2-(2-(2,2,2-trifluoroethoxy)phenoxy)ethyl)amino)propyl)indoline-7-carboxamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: hydrogen; palladium 10% on activated carbon / methanol; tetrahydrofuran / 48 h / 40 °C 2: sodium tris(acetoxy)borohydride / 1,2-dichloro-ethane / 12 h / 20 °C 3: sodium hydroxide; water / methanol / 2 h / 20 °C 4: sodium hydroxide; water; dihydrogen peroxide / dimethyl sulfoxide / 0 - 25 °C View Scheme |

-
-
160970-64-9
1-(3-hydroxypropyl)-5-(2-((2-(2-(2,2,2-trifluoroethoxy)phenoxy)ethyl)amino)propyl)indoline-7-carboxamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hydroxide; water / methanol / 2 h / 20 °C 2: sodium hydroxide; water; dihydrogen peroxide / dimethyl sulfoxide / 0 - 25 °C View Scheme |
-
-
1338365-54-0
5-(2-aminopropyl)-1-[3-(benzoyloxy)propyl]-2,3-dihydro-7-cyano-1H-indole

-
-
160970-64-9
1-(3-hydroxypropyl)-5-(2-((2-(2-(2,2,2-trifluoroethoxy)phenoxy)ethyl)amino)propyl)indoline-7-carboxamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium tris(acetoxy)borohydride / 1,2-dichloro-ethane / 12 h / 20 °C 2: sodium hydroxide; water / methanol / 2 h / 20 °C 3: sodium hydroxide; water; dihydrogen peroxide / dimethyl sulfoxide / 0 - 25 °C View Scheme |
-
-
160970-64-9
1-(3-hydroxypropyl)-5-(2-((2-(2-(2,2,2-trifluoroethoxy)phenoxy)ethyl)amino)propyl)indoline-7-carboxamide

-
-
160970-54-7
silodosin

| Conditions | Yield |
|---|---|
| In ethyl acetate at 15 - 55℃; | 7.2g |
Silodosin Chemical Properties
Molecule structure of Silodosin (CAS NO.160970-64-9):
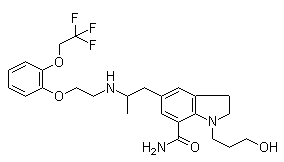
IUPAC Name: 1-(3-Hydroxypropyl)-5-[(2R)-2-[2-[2-(2,2,2-trifluoroethoxy)phenoxy]ethylamino]propyl]-2,3-dihydroindole-7-carboxamide
Molecular Weight: 495.53449 g/mol
Molecular Formula: C25H32F3N3O4
Density: 1.249 g/cm3
Boiling Point: 601.4 °C at 760 mmHg
Flash Point: 317.5 °C
Index of Refraction: 1.552
Molar Refractivity: 126.82 cm3
Molar Volume: 396.6 cm3
Surface Tension: 45.5 dyne/cm
Enthalpy of Vaporization: 94.11 kJ/mol
Vapour Pressure: 2.58E-15 mmHg at 25 °C
XLogP3-AA: 3.6
H-Bond Donor: 3
H-Bond Acceptor: 9
Rotatable Bond Count: 13
Tautomer Count: 2
Exact Mass: 495.234491
MonoIsotopic Mass: 495.234491
Topological Polar Surface Area: 97
Heavy Atom Count: 35
Canonical SMILES: CC(CC1=CC2=C(C(=C1)C(=O)N)N(CC2)CCCO)NCCOC3=CC=CC=C3OCC(F)(F)F
Isomeric SMILES: C[C@H](CC1=CC2=C(C(=C1)C(=O)N)N(CC2)CCCO)NCCOC3=CC=CC=C3OCC(F)(F)F
InChI: InChI=1S/C25H32F3N3O4/c1-17(30-8-12-34-21-5-2-3-6-22(21)35-16-25(26,27)28)13-18-14-19-7-10-31(9-4-11-32)23(19)20(15-18)24(29)33/h2-3,5-6,14-15,17,30,32H,4,7-13,16H2,1H3,(H2,29,33)/t17-/m1/s1
InChIKey: PNCPYILNMDWPEY-QGZVFWFLSA-N
Silodosin History
In May 2006, Silodosin (CAS NO.160970-64-9) received its first marketing approval under the tradename Urief, which is jointly marketed by Daiichi Sankyo Pharmaceutical Co., Ltd and Kissei Pharmaceutical Co., Ltd.. In 2004, Kissei licensed the US, Canadian, and Mexican rights for silodosin to Watson Pharmaceuticals, Inc.. On February 12, 2008, Watson announced that the New Drug Application submitted to the United States Food and Drug Administration for silodosin has been accepted for filing. FDA approved this drug on October 9th, 2008. Silodosin is marketed under the tradename Rapaflo.
Silodosin Specification
Silodosin (CAS NO.160970-64-9) is also named as Rapaflo ; Rapflo ; UNII-CUZ39LUY82 ; (-)-1-(3-Hydroxypropyl)-5-((2R)-2-((2-(2-(2,2,2-trifluoroethoxy)phenoxy)ethyl)amino)propyl)-2,3-dihydro-1H-indole-7-carboxamide ; 160970-54-7 .
Related Products
- Silodosin
- Silodosin
- 16097-49-7
- 16097-60-2
- 160976-02-3
- 16097-61-3
- 16097-62-4
- 16097-63-5
- 16097-64-6
- 160977-92-4
- 160982-09-2
- 160982-10-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View