-
Name
TABERSONINE
- EINECS 224-615-0
- CAS No. 4429-63-4
- Article Data17
- CAS DataBase
- Density 1.27 g/cm3
- Solubility
- Melting Point 196 °C
- Formula C21H24N2O2
- Boiling Point 488.7 °C at 760 mmHg
- Molecular Weight 336.434
- Flash Point 249.4 °C
- Transport Information
- Appearance Yellow to brownish powder, pungent odour
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 1H-Indolizino[8,1-cd]carbazole-5-carboxylicacid, 3a-ethyl-3a,4,6,11,12,13a-hexahydro-, methyl ester (7CI);Aspidospermidine-3-carboxylic acid, 2,3,6,7-tetradehydro-, methyl ester, (5a,12b,19a)-;Tabersonine (8CI);(-)-Tabersonine;Tabersonin;
- PSA 41.57000
- LogP 3.29710
Synthetic route
-
-
55856-70-7
19-Iodo-tabersonine

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; hydrogen; nickel In methanol at 0℃; for 0.0833333h; | 90% |
-
-
62414-79-3
methyl (2S,3aR,3a1S,10bR)-3a-ethyl-2-hydroxy-2,3,3a,3a1,4,6,11,12-octahydro-1H-indolizino-[8,1-cd]carbazole-5-carboxylate

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| With triphenylphosphine In tetrachloromethane; acetonitrile at 70℃; for 0.5h; | 73% |
| With triphenylphosphine In tetrachloromethane; acetonitrile at 70℃; for 0.5h; | 71% |
| With triphenylphosphine In tetrachloromethane; acetonitrile at 70℃; for 0.5h; Inert atmosphere; | 64% |
-
-
17640-15-2
methyl cyanoformate

-
-
32975-46-5
(-)-1,2,14,15-tetradehydroaspidospermidine

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| Stage #1: (-)-1,2,14,15-tetradehydroaspidospermidine With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at -78 - 0℃; for 1h; Inert atmosphere; Stage #2: methyl cyanoformate In tetrahydrofuran; hexane at -78℃; for 1h; Inert atmosphere; | 73% |
| With lithium diisopropyl amide In tetrahydrofuran at -78℃; | 73% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 30℃; Irradiation; Title compound not separated from byproducts; | A n/a B 10% |
-
-
62414-79-3, 63490-95-9, 74165-08-5, 80695-59-6, 80695-62-1, 95042-67-4
(S)-3a-Ethyl-2-hydroxy-2,3,3a,4,6,11,12,12b-octahydro-1H-6,12a-diaza-indeno[7,1-cd]fluorene-5-carboxylic acid methyl ester

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| With tetrachloromethane; triphenylphosphine In acetonitrile |
-
-
67249-34-7
tabersonine N4-oxide

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| With iron(II) sulfate for 0.25h; Heating; | 0.5 mg |
-
-
41787-54-6
16-epi-19-S-vindolinine

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 70 percent / 10percent Na2CO3, I2 / tetrahydrofuran; H2O / 1 h / Ambient temperature 2: 90 percent / H2, NaOH / Raney Nickel / methanol / 0.08 h / 0 °C View Scheme |
-
-
80642-07-5
5-chloro-2-ethyl-(4S)-hydroxypentanal lactol

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1.) boric acid, 2.) triethylamine / 1.) methanol, reflux, 24 h, 2.) methanol, reflux, 12 h 2: 71 percent / triphenylphosphine / acetonitrile; CCl4 / 0.5 h / 70 °C View Scheme |
-
-
66859-22-1
methyl 1,2,3,4,5,6-hexahydroazepino<4,5-b>indole-5-carboxylate

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1.) boric acid, 2.) triethylamine / 1.) methanol, reflux, 24 h, 2.) methanol, reflux, 12 h 2: 71 percent / triphenylphosphine / acetonitrile; CCl4 / 0.5 h / 70 °C View Scheme |
-
-
124293-81-8
1-(benzenesulfonyl)-2-methyl-1H-indole-3-carbaldehyde

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1.1: titanium(IV) tetraethanolate / tetrahydrofuran / 24 h / 20 °C / Inert atmosphere 2.1: lithium hexamethyldisilazane / tetrahydrofuran / 1 h / -78 °C / Inert atmosphere 2.2: 2 h / -78 °C / Inert atmosphere 3.1: lithium hexamethyldisilazane / N,N-dimethyl-formamide / 0.67 h / -15 °C / Inert atmosphere 3.2: 2 h / -15 °C / Inert atmosphere 4.1: diisobutylaluminium hydride / dichloromethane / 1 h / 0 °C / Inert atmosphere 5.1: Dess-Martin periodane; sodium hydrogencarbonate / dichloromethane / 3 h / 0 - 20 °C / Inert atmosphere 6.1: n-butyllithium / tetrahydrofuran; hexane / 0.67 h / -78 - 0 °C / Inert atmosphere 6.2: 2 h / -78 - 20 °C / Inert atmosphere 7.1: Hoveyda-Grubbs catalyst second generation / dichloromethane / 48 h / 20 °C / Inert atmosphere 8.1: hydrogenchloride / methanol; water / 0 °C / Inert atmosphere 9.1: sodium carbonate / ethanol / 24 h / 85 °C / Inert atmosphere 10.1: potassium tert-butylate / tetrahydrofuran / 2 h / 0 °C / Inert atmosphere 11.1: n-butyllithium; diisopropylamine / tetrahydrofuran; hexane / 1 h / -78 - 0 °C / Inert atmosphere 11.2: 1 h / -78 °C / Inert atmosphere View Scheme |

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: lithium hexamethyldisilazane / tetrahydrofuran / 1 h / -78 °C / Inert atmosphere 1.2: 2 h / -78 °C / Inert atmosphere 2.1: lithium hexamethyldisilazane / N,N-dimethyl-formamide / 0.67 h / -15 °C / Inert atmosphere 2.2: 2 h / -15 °C / Inert atmosphere 3.1: diisobutylaluminium hydride / dichloromethane / 1 h / 0 °C / Inert atmosphere 4.1: Dess-Martin periodane; sodium hydrogencarbonate / dichloromethane / 3 h / 0 - 20 °C / Inert atmosphere 5.1: n-butyllithium / tetrahydrofuran; hexane / 0.67 h / -78 - 0 °C / Inert atmosphere 5.2: 2 h / -78 - 20 °C / Inert atmosphere 6.1: Hoveyda-Grubbs catalyst second generation / dichloromethane / 48 h / 20 °C / Inert atmosphere 7.1: hydrogenchloride / methanol; water / 0 °C / Inert atmosphere 8.1: sodium carbonate / ethanol / 24 h / 85 °C / Inert atmosphere 9.1: potassium tert-butylate / tetrahydrofuran / 2 h / 0 °C / Inert atmosphere 10.1: n-butyllithium; diisopropylamine / tetrahydrofuran; hexane / 1 h / -78 - 0 °C / Inert atmosphere 10.2: 1 h / -78 °C / Inert atmosphere View Scheme |
-
-
1451192-91-8
C26H32N2O5S2

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: lithium hexamethyldisilazane / N,N-dimethyl-formamide / 0.67 h / -15 °C / Inert atmosphere 1.2: 2 h / -15 °C / Inert atmosphere 2.1: diisobutylaluminium hydride / dichloromethane / 1 h / 0 °C / Inert atmosphere 3.1: Dess-Martin periodane; sodium hydrogencarbonate / dichloromethane / 3 h / 0 - 20 °C / Inert atmosphere 4.1: n-butyllithium / tetrahydrofuran; hexane / 0.67 h / -78 - 0 °C / Inert atmosphere 4.2: 2 h / -78 - 20 °C / Inert atmosphere 5.1: Hoveyda-Grubbs catalyst second generation / dichloromethane / 48 h / 20 °C / Inert atmosphere 6.1: hydrogenchloride / methanol; water / 0 °C / Inert atmosphere 7.1: sodium carbonate / ethanol / 24 h / 85 °C / Inert atmosphere 8.1: potassium tert-butylate / tetrahydrofuran / 2 h / 0 °C / Inert atmosphere 9.1: n-butyllithium; diisopropylamine / tetrahydrofuran; hexane / 1 h / -78 - 0 °C / Inert atmosphere 9.2: 1 h / -78 °C / Inert atmosphere View Scheme |
-
-
1451192-86-1
C29H36N2O5S2

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: diisobutylaluminium hydride / dichloromethane / 1 h / 0 °C / Inert atmosphere 2.1: Dess-Martin periodane; sodium hydrogencarbonate / dichloromethane / 3 h / 0 - 20 °C / Inert atmosphere 3.1: n-butyllithium / tetrahydrofuran; hexane / 0.67 h / -78 - 0 °C / Inert atmosphere 3.2: 2 h / -78 - 20 °C / Inert atmosphere 4.1: Hoveyda-Grubbs catalyst second generation / dichloromethane / 48 h / 20 °C / Inert atmosphere 5.1: hydrogenchloride / methanol; water / 0 °C / Inert atmosphere 6.1: sodium carbonate / ethanol / 24 h / 85 °C / Inert atmosphere 7.1: potassium tert-butylate / tetrahydrofuran / 2 h / 0 °C / Inert atmosphere 8.1: n-butyllithium; diisopropylamine / tetrahydrofuran; hexane / 1 h / -78 - 0 °C / Inert atmosphere 8.2: 1 h / -78 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 5 steps 1.1: diisobutylaluminium hydride / dichloromethane / -78 °C 1.2: 0 - 20 °C 2.1: Methylenetriphenylphosphorane / -78 - 20 °C 2.2: 20 °C 3.1: hydrogenchloride / water 3.2: Reflux 4.1: potassium tert-butylate / tetrahydrofuran / 0 °C 5.1: lithium diisopropyl amide / tetrahydrofuran / -78 °C View Scheme |
-
-
1451192-92-9
C28H36N2O4S2

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: Dess-Martin periodane; sodium hydrogencarbonate / dichloromethane / 3 h / 0 - 20 °C / Inert atmosphere 2.1: n-butyllithium / tetrahydrofuran; hexane / 0.67 h / -78 - 0 °C / Inert atmosphere 2.2: 2 h / -78 - 20 °C / Inert atmosphere 3.1: Hoveyda-Grubbs catalyst second generation / dichloromethane / 48 h / 20 °C / Inert atmosphere 4.1: hydrogenchloride / methanol; water / 0 °C / Inert atmosphere 5.1: sodium carbonate / ethanol / 24 h / 85 °C / Inert atmosphere 6.1: potassium tert-butylate / tetrahydrofuran / 2 h / 0 °C / Inert atmosphere 7.1: n-butyllithium; diisopropylamine / tetrahydrofuran; hexane / 1 h / -78 - 0 °C / Inert atmosphere 7.2: 1 h / -78 °C / Inert atmosphere View Scheme |
-
-
1451192-88-3
C28H34N2O4S2

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: n-butyllithium / tetrahydrofuran; hexane / 0.67 h / -78 - 0 °C / Inert atmosphere 1.2: 2 h / -78 - 20 °C / Inert atmosphere 2.1: Hoveyda-Grubbs catalyst second generation / dichloromethane / 48 h / 20 °C / Inert atmosphere 3.1: hydrogenchloride / methanol; water / 0 °C / Inert atmosphere 4.1: sodium carbonate / ethanol / 24 h / 85 °C / Inert atmosphere 5.1: potassium tert-butylate / tetrahydrofuran / 2 h / 0 °C / Inert atmosphere 6.1: n-butyllithium; diisopropylamine / tetrahydrofuran; hexane / 1 h / -78 - 0 °C / Inert atmosphere 6.2: 1 h / -78 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1.1: Methylenetriphenylphosphorane / -78 - 20 °C 1.2: 20 °C 2.1: hydrogenchloride / water 2.2: Reflux 3.1: potassium tert-butylate / tetrahydrofuran / 0 °C 4.1: lithium diisopropyl amide / tetrahydrofuran / -78 °C View Scheme |
-
-
1451192-93-0
C29H36N2O3S2

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: Hoveyda-Grubbs catalyst second generation / dichloromethane / 48 h / 20 °C / Inert atmosphere 2.1: hydrogenchloride / methanol; water / 0 °C / Inert atmosphere 3.1: sodium carbonate / ethanol / 24 h / 85 °C / Inert atmosphere 4.1: potassium tert-butylate / tetrahydrofuran / 2 h / 0 °C / Inert atmosphere 5.1: n-butyllithium; diisopropylamine / tetrahydrofuran; hexane / 1 h / -78 - 0 °C / Inert atmosphere 5.2: 1 h / -78 °C / Inert atmosphere View Scheme |
-
-
1451192-89-4
C27H32N2O3S2

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: hydrogenchloride / methanol; water / 0 °C / Inert atmosphere 2.1: sodium carbonate / ethanol / 24 h / 85 °C / Inert atmosphere 3.1: potassium tert-butylate / tetrahydrofuran / 2 h / 0 °C / Inert atmosphere 4.1: n-butyllithium; diisopropylamine / tetrahydrofuran; hexane / 1 h / -78 - 0 °C / Inert atmosphere 4.2: 1 h / -78 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1.1: hydrogenchloride / water 1.2: Reflux 2.1: potassium tert-butylate / tetrahydrofuran / 0 °C 3.1: lithium diisopropyl amide / tetrahydrofuran / -78 °C View Scheme |
-
-
1451192-94-1
C23H24N2O2S

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sodium carbonate / ethanol / 24 h / 85 °C / Inert atmosphere 2.1: potassium tert-butylate / tetrahydrofuran / 2 h / 0 °C / Inert atmosphere 3.1: n-butyllithium; diisopropylamine / tetrahydrofuran; hexane / 1 h / -78 - 0 °C / Inert atmosphere 3.2: 1 h / -78 °C / Inert atmosphere View Scheme |
-
-
1451192-90-7
C25H28N2O3S

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: potassium tert-butylate / tetrahydrofuran / 2 h / 0 °C / Inert atmosphere 2.1: n-butyllithium; diisopropylamine / tetrahydrofuran; hexane / 1 h / -78 - 0 °C / Inert atmosphere 2.2: 1 h / -78 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: potassium tert-butylate / tetrahydrofuran / 0 °C 2: lithium diisopropyl amide / tetrahydrofuran / -78 °C View Scheme |

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: sodium hydride; 1H-imidazole / tetrahydrofuran 2.1: lithium hexamethyldisilazane / tetrahydrofuran / -78 °C 3.1: N,N-dimethyl-formamide / -78 - 20 °C 4.1: Methylenetriphenylphosphorane / -78 - 20 °C 4.2: 20 °C 5.1: hydrogenchloride / water 5.2: Reflux 6.1: potassium tert-butylate / tetrahydrofuran / 0 °C 7.1: lithium diisopropyl amide / tetrahydrofuran / -78 °C View Scheme | |
| Multi-step reaction with 8 steps 1.1: sodium hydride; 1H-imidazole / tetrahydrofuran 2.1: lithium hexamethyldisilazane / tetrahydrofuran / -78 °C 3.1: lithium hexamethyldisilazane / N,N-dimethyl-formamide / -78 - 20 °C 4.1: diisobutylaluminium hydride / dichloromethane / -78 °C 4.2: 0 - 20 °C 5.1: Methylenetriphenylphosphorane / -78 - 20 °C 5.2: 20 °C 6.1: hydrogenchloride / water 6.2: Reflux 7.1: potassium tert-butylate / tetrahydrofuran / 0 °C 8.1: lithium diisopropyl amide / tetrahydrofuran / -78 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: titanium(IV) tetraethanolate / tetrahydrofuran / 24 h / 70 °C 2.1: sodium hydride; 1H-imidazole / tetrahydrofuran 3.1: lithium hexamethyldisilazane / tetrahydrofuran / -78 °C 4.1: N,N-dimethyl-formamide / -78 - 20 °C 5.1: Methylenetriphenylphosphorane / -78 - 20 °C 5.2: 20 °C 6.1: hydrogenchloride / water 6.2: Reflux 7.1: potassium tert-butylate / tetrahydrofuran / 0 °C 8.1: lithium diisopropyl amide / tetrahydrofuran / -78 °C View Scheme | |
| Multi-step reaction with 9 steps 1.1: titanium(IV) tetraethanolate / tetrahydrofuran / 24 h / 70 °C 2.1: sodium hydride; 1H-imidazole / tetrahydrofuran 3.1: lithium hexamethyldisilazane / tetrahydrofuran / -78 °C 4.1: lithium hexamethyldisilazane / N,N-dimethyl-formamide / -78 - 20 °C 5.1: diisobutylaluminium hydride / dichloromethane / -78 °C 5.2: 0 - 20 °C 6.1: Methylenetriphenylphosphorane / -78 - 20 °C 6.2: 20 °C 7.1: hydrogenchloride / water 7.2: Reflux 8.1: potassium tert-butylate / tetrahydrofuran / 0 °C 9.1: lithium diisopropyl amide / tetrahydrofuran / -78 °C View Scheme |

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: lithium hexamethyldisilazane / tetrahydrofuran / -78 °C 2.1: N,N-dimethyl-formamide / -78 - 20 °C 3.1: Methylenetriphenylphosphorane / -78 - 20 °C 3.2: 20 °C 4.1: hydrogenchloride / water 4.2: Reflux 5.1: potassium tert-butylate / tetrahydrofuran / 0 °C 6.1: lithium diisopropyl amide / tetrahydrofuran / -78 °C View Scheme | |
| Multi-step reaction with 7 steps 1.1: lithium hexamethyldisilazane / tetrahydrofuran / -78 °C 2.1: lithium hexamethyldisilazane / N,N-dimethyl-formamide / -78 - 20 °C 3.1: diisobutylaluminium hydride / dichloromethane / -78 °C 3.2: 0 - 20 °C 4.1: Methylenetriphenylphosphorane / -78 - 20 °C 4.2: 20 °C 5.1: hydrogenchloride / water 5.2: Reflux 6.1: potassium tert-butylate / tetrahydrofuran / 0 °C 7.1: lithium diisopropyl amide / tetrahydrofuran / -78 °C View Scheme |

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: lithium hexamethyldisilazane / N,N-dimethyl-formamide / -78 - 20 °C 2.1: diisobutylaluminium hydride / dichloromethane / -78 °C 2.2: 0 - 20 °C 3.1: Methylenetriphenylphosphorane / -78 - 20 °C 3.2: 20 °C 4.1: hydrogenchloride / water 4.2: Reflux 5.1: potassium tert-butylate / tetrahydrofuran / 0 °C 6.1: lithium diisopropyl amide / tetrahydrofuran / -78 °C View Scheme |

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: N,N-dimethyl-formamide / -78 - 20 °C 2.1: Methylenetriphenylphosphorane / -78 - 20 °C 2.2: 20 °C 3.1: hydrogenchloride / water 3.2: Reflux 4.1: potassium tert-butylate / tetrahydrofuran / 0 °C 5.1: lithium diisopropyl amide / tetrahydrofuran / -78 °C View Scheme |

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: tetrabutyl ammonium fluoride / tetrahydrofuran / 8 h / 0 - 20 °C / Inert atmosphere 2: triphenylphosphine / acetonitrile; tetrachloromethane / 0.5 h / 70 °C / Inert atmosphere View Scheme |

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: T. iboga precondylocarpine acetate synthase 1; FAD / aq. buffer / 2 h / 37 °C / pH 9.5 / Enzymatic reaction 2: NADPH / aq. buffer / 1 h / 37 °C / pH 9.5 / Enzymatic reaction 3: NADPH / aq. buffer / pH 9.5 / Enzymatic reaction View Scheme |

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NADPH / aq. buffer / 1 h / 37 °C / pH 9.5 / Enzymatic reaction 2: NADPH / aq. buffer / pH 9.5 / Enzymatic reaction View Scheme |

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| With NADPH In aq. buffer pH=9.5; Reagent/catalyst; Enzymatic reaction; |

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: pyridine / 20 °C 1.2: 4 h / 20 °C 2.1: flavin adenine dinucleotide; precondylocarpine acetate synthase; dehydroprecondyiocarpine acetate synthase; tabersonine synthase / methanol / pH 8.5 / Enzymatic reaction View Scheme | |
| Multi-step reaction with 3 steps 1.1: pyridine / 20 °C 1.2: 4 h / 20 °C 2.1: platinum; oxygen / ethyl acetate / 20 °C 3.1: NADPH; dehydroprecondyiocarpine acetate synthase; tabersonine synthase / methanol / 1 h / 37 °C / pH 7.5 / Enzymatic reaction View Scheme | |
| Multi-step reaction with 3 steps 1.1: pyridine / 20 °C 1.2: 4 h / 20 °C 2.1: flavin adenine dinucleotide; precondylocarpine acetate synthase; dehydroprecondyiocarpine acetate synthase / methanol / pH 8.5 / Enzymatic reaction 3.1: NADPH; dehydroprecondyiocarpine acetate synthase; tabersonine synthase / methanol / 1 h / 37 °C / pH 7.5 / Enzymatic reaction View Scheme |

-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| With dehydroprecondyiocarpine acetate synthase; precondylocarpine acetate synthase; tabersonine synthase; flavin adenine dinucleotide In methanol pH=8.5; Enzymatic reaction; | |
| Multi-step reaction with 2 steps 1: platinum; oxygen / ethyl acetate / 20 °C 2: NADPH; dehydroprecondyiocarpine acetate synthase; tabersonine synthase / methanol / 1 h / 37 °C / pH 7.5 / Enzymatic reaction View Scheme |
-
-
4429-63-4
tabersonine

-
-
110456-33-2
dihydro-2β,16β tabersonine

| Conditions | Yield |
|---|---|
| With sodium cyanoborohydride; acetic acid In methanol; water at 20℃; for 20h; Inert atmosphere; | 100% |
| With hydrogenchloride; zinc In methanol for 3h; Heating; | 76% |
| With sodium cyanoborohydride In propionic acid for 0.166667h; Ambient temperature; |
-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| With N-iodo-succinimide; trifluoroacetic acid at 20℃; for 3h; | 98% |
| With N-iodo-succinimide |

| Conditions | Yield |
|---|---|
| Stage #1: tabersonine With ammonium hydroxide In Petroleum ether at 20℃; for 0.5h; Stage #2: With palladium on activated charcoal; hydrogen In methanol at 20℃; under 760.051 Torr; for 7h; Solvent; Temperature; | 95.4% |
| With platinum(IV) oxide; hydrogen In ethyl acetate for 16h; Inert atmosphere; | 81% |
| With platinum(IV) oxide; hydrogen In ethyl acetate | 81% |
| With hydrogen; palladium | |
| With palladium 10% on activated carbon; hydrogen In tetrahydrofuran at 35 - 45℃; under 3000.3 - 3750.38 Torr; |
-
-
4429-63-4
tabersonine

-
-
100044-35-7
decarbomethoxytabersonine

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 110℃; | 94% |
-
-
4429-63-4
tabersonine

-
-
93771-99-4
(-)-17-hydroxytabersonine

| Conditions | Yield |
|---|---|
| With triphenylphosphine In tetrachloromethane; acetonitrile at 70℃; for 0.5h; | 93% |
| With benzeneseleninic anhydride In benzene at 60℃; for 0.5h; | 93% |

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol at 50℃; for 24h; | A 3% B 92% |

| Conditions | Yield |
|---|---|
| Stage #1: tabersonine With dmap; sodium hydride In tetrahydrofuran; N,N-dimethyl-formamide at 0℃; for 1h; Stage #2: 2,2,2-Trichloroethyl chloroformate In tetrahydrofuran; N,N-dimethyl-formamide at 20℃; for 3h; | 92% |
-
-
4429-63-4
tabersonine

-
-
73749-08-3
methyl 15-bromo-2,3,6,7-tetrahydro-(5α,12β,19α)-aspidospermidine-3-carboxylate

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; acetic acid at 20℃; for 4h; | 91% |
| With N-Bromosuccinimide In tetrahydrofuran |
-
-
4429-63-4
tabersonine

-
-
79820-33-0
14,15-didehydro-16-hydroxy-<3H>indole

| Conditions | Yield |
|---|---|
| With oxygen; rose bengal; triethyl phosphite In methanol for 0.333333h; Irradiation; | 88% |
| With acetic buffer; ozone at 25℃; | 78% |
| Multi-step reaction with 2 steps 1: 38 percent / m-chloroperbenzoic acid / benzene / 24 h / Ambient temperature 2: 73 percent / Raney Ni / acetone View Scheme |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid at 20℃; for 24h; | 85% |
-
-
4429-63-4
tabersonine

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile) In dichloromethane for 5h; Reflux; Inert atmosphere; | 81% |
-
-
4429-63-4
tabersonine

-
A
-
62414-79-3
methyl (2S,3aR,3a1S,10bR)-3a-ethyl-2-hydroxy-2,3,3a,3a1,4,6,11,12-octahydro-1H-indolizino-[8,1-cd]carbazole-5-carboxylate

-
B
-
63490-95-9
7-hydroxy-2,3-didehydro-aspidospermidine-3-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| Hydroboration, Oxidation; | A 20% B 80% |

| Conditions | Yield |
|---|---|
| With potassiuim nitrosodisulfonate; acetic acid In N,N-dimethyl-formamide for 96h; Ambient temperature; | A 8% B 78% |

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide; trifluoroacetic acid at 20℃; for 4h; | A 68% B 7% |
-
-
4429-63-4
tabersonine

-
A
-
78346-69-7
(3aR,10bR,12bS)-3a-Ethyl-3a,4,5,11,12,12b-hexahydro-1H,6H-6,12a-diaza-indeno[7,1-cd]fluoren-5a-ol

-
B
-
32975-46-5
(-)-1,2,14,15-tetradehydroaspidospermidine

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 105℃; for 0.166667h; | A 65% B 28% |
| With hydrogenchloride at 105℃; for 0.166667h; | A 49% B 41% |
-
-
67-56-1
methanol

-
-
4429-63-4
tabersonine

-
-
73232-83-4
dichloro-10,12 dihydro-2,16 hydroxy-16 methoxy-2 tabersonine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; 3-chloro-benzenecarboperoxoic acid for 24h; Ambient temperature; | 60% |
| With hydrogenchloride; MCPB |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; 3-chloro-benzenecarboperoxoic acid In methanol for 24h; Ambient temperature; | A 30% B 60% |
| In methanol for 24h; Product distribution; Ambient temperature; diferent amount of hydrochloric acid and m-chloroperbenzoic acid, other derivative of tabersonine; | A 30% B 60% |
| With 3,3-dimethyldioxirane; trifluoroacetic anhydride In dichloromethane; acetone at 0℃; for 10h; | A 29% B 27% |
-
-
64-17-5
ethanol

-
-
4429-63-4
tabersonine

-
-
77250-38-5
(3aR,5R,5aS,10bR,12bS)-7,9-Dichloro-5a-ethoxy-3a-ethyl-5-hydroxy-3a,4,5,5a,6,11,12,12b-octahydro-1H-6,12a-diaza-indeno[7,1-cd]fluorene-5-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| With hydrogenchloride; 3-chloro-benzenecarboperoxoic acid for 24h; Ambient temperature; | 60% |
-
-
4429-63-4
tabersonine

-
B
-
69754-29-6
16-epi-14,15-dehydro-10-chloro vincamine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; 3-chloro-benzenecarboperoxoic acid In methanol for 24h; Ambient temperature; | A 25% B 55% |
-
-
4429-63-4
tabersonine

-
-
407-25-0
trifluoroacetic anhydride

-
-
137993-96-5
(3S,3aS,10bR)-3a-Ethyl-3-hydroxy-2,6-bis-(2,2,2-trifluoro-acetyl)-3a,4,6,11,12,12b-hexahydro-3H-6,12a-diaza-indeno[7,1-cd]fluorene-5-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| With pyridine for 12h; Ambient temperature; | 47% |
-
-
4429-63-4
tabersonine

-
B
-
62414-79-3
methyl (2S,3aR,3a1S,10bR)-3a-ethyl-2-hydroxy-2,3,3a,3a1,4,6,11,12-octahydro-1H-indolizino-[8,1-cd]carbazole-5-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: tabersonine With sodium tetrahydroborate; boron trifluoride diethyl etherate In tetrahydrofuran at 0℃; for 2.5h; Stage #2: With sodium hydroxide; dihydrogen peroxide In tetrahydrofuran at 60℃; for 0.75h; Further stages.; | A 6.6% B 41.9% |
-
-
4429-63-4
tabersonine

-
A
-
128773-80-8
14β-Bromo 15α-fluoro vincadifformine

-
B
-
128773-82-0
14β-Fluoro 15α-bromo vincadifformine

| Conditions | Yield |
|---|---|
| With hydrogen fluoride; bromine; antimony pentafluoride at -35℃; for 0.75h; Mechanism; | A 27% B 41.4% |
-
-
4429-63-4
tabersonine

-
-
80249-96-3
16-hydroxy-tabersonine-N-oxide

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In benzene | 40% |
| With 3,3-dimethyldioxirane In dichloromethane; acetone at 4 - 5℃; for 24h; | 31% |
-
-
4429-63-4
tabersonine

-
-
82690-89-9
(3aR,5R,10bR,12aR,12bS)-3a-Ethyl-5-hydroxy-12a-oxy-3a,4,5,11,12,12b-hexahydro-1H-6,12a-diaza-indeno[7,1-cd]fluorene-5-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In benzene for 24h; Ambient temperature; | 38% |
-
-
4429-63-4
tabersonine

-
A
-
128773-79-5
15α-Fluoro 14β-hydroxy vincadifformine

-
B
-
128773-78-4
14α-Fluoro 15β-hydroxy vincadifformine

-
C
-
128779-74-8
14β-Fluoro 15α-hydroxy vincadifformine

| Conditions | Yield |
|---|---|
| With hydrogen fluoride; dihydrogen peroxide; antimony pentafluoride at -35℃; for 1h; Mechanism; | A 21% B 22% C 35% |

-
-
4429-63-4
tabersonine

-
A
-
74947-48-1
C21H22N2O5

-
B
-
32790-09-3
Δ14-vincamine

-
C
-
32790-10-6
14-epi-Δ17-vincamine

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In benzene at 20℃; for 24h; | A 5% B 29% C 31% |

-
-
4429-63-4
tabersonine

-
A
-
112794-42-0
(3aR,5R,10bR,12bS)-5-Aminooxy-3a-ethyl-3a,4,5,11,12,12b-hexahydro-1H-6,12a-diaza-indeno[7,1-cd]fluorene-5-carboxylic acid methyl ester

-
B
-
112794-43-1
C21H27N3O7S

| Conditions | Yield |
|---|---|
| With potassiuim nitrosodisulfonate; acetic acid In N,N-dimethyl-formamide for 2h; Ambient temperature; | A 27% B 6% |
Tabersonine Chemical Properties
Molecular structure of Tabersonine (CAS NO.4429-63-4) is:
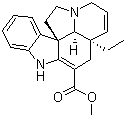
Product Name: Tabersonine
CAS Registry Number: 4429-63-4
Molecular Weight: 336.42746 [g/mol]
Molecular Formula: C21H24N2O2
XLogP3-AA: 3.4
H-Bond Donor: 1
H-Bond Acceptor: 4
EINECS: 224-615-0
Index of Refraction: 1.651
Molar Refractivity: 96.78 cm3
Molar Volume: 264.7 cm3
Surface Tension: 55.7 dyne/cm
Density: 1.27 g/cm3
Flash Point: 249.4 °C
Enthalpy of Vaporization: 75.48 kJ/mol
Boiling Point: 488.7 °C at 760 mmHg
Vapour Pressure: 1.06E-09 mmHg at 25°C
Classification Code: Drug / Therapeutic Agent
Tabersonine Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intravenous | 100mg/kg (100mg/kg) | Annales Pharmaceutiques Francaises. Vol. 13, Pg. 123, 1955. |
Tabersonine Specification
Tabersonine , its cas register number is 4429-63-4. It also can be called Tabersonin ; Aspidospermidine-3-carboxylic acid, 2,3,6,7-tetradehydro-,methyl ester, (5alpha,12beta,19alpha)- ; Aspidospermidine-3-carboxylic acid, 2,3,6,7-tetrahydro-,methyl ester, (5-alpha,12-beta,19-alpha)- .It is a yellow to brownish powder with pungent odour .
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View