-
Name
TRIPHENYLBORANE
- EINECS 213-504-2
- CAS No. 960-71-4
- Article Data117
- CAS DataBase
- Density 1.042 g/cm3
- Solubility Soluble in aromatic solvents. Insoluble in water.
- Melting Point 145 °C(lit.)
- Formula C18H15B
- Boiling Point 348 °C at 760 mmHg
- Molecular Weight 242.128
- Flash Point 161.1 °C
- Transport Information UN 1993 3/PG 2
- Appearance white crystals
- Safety 16-26-33-36-24/25-22
- Risk Codes 11-36/37/38-22-19
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, F
F
- Synonyms Borine,triphenyl- (6CI);Triphenylborine;Triphenylboron;
- PSA 0.00000
- LogP 2.20280
Triphenylborane Specification
The IUPAC name of this chemical is Triphenylborane. With the CAS registry number 960-71-4 and EINECS registry number 213-504-2, it is also named as Borane, triphenyl-. In addition, the molecular formula is C18H15B and the molecular weight is 242.12. It is a kind of white crystalline solid and belongs to the classes of Boron; Precursors by Metal; Vapor Deposition Precursors; BoronAlphabetic; Micro/Nanoelectronics; Solution Deposition Precursors; TP - TZ. And it is soluble in aromatic solvents.
Physical properties about this chemical are: (1)ACD/LogP: 5.35; (2)# of Rule of 5 Violations: 1; (3)ACD/LogD (pH 5.5): 5.35; (4)ACD/LogD (pH 7.4): 5.35; (5)ACD/BCF (pH 5.5): 6880.35; (6)ACD/BCF (pH 7.4): 6880.35; (7)ACD/KOC (pH 5.5): 19433.58; (8)ACD/KOC (pH 7.4): 19433.58 ; (9)#Freely Rotating Bonds: 3; (10)Polar Surface Area: 0 Å2; (11)Index of Refraction: 1.601; (12)Molar Refractivity: 79.64 cm3; (13)Molar Volume: 232.4 cm3; (14)Polarizability: 31.57 ×10-24cm3; (15)Surface Tension: 40.2 dyne/cm; (16)Density: 1.04 g/cm3; (17)Flash Point: 161.1 °C; (18)Enthalpy of Vaporization: 56.89 kJ/mol; (19)Boiling Point: 348 °C at 760 mmHg; (20)Vapour Pressure: 0.000104 mmHg at 25°C.
Preparation of Triphenylborane: It is typically made with boron trifluoride diethyl etherate and the Grignard reagent, phenylmagnesium bromide. The equation is as follows: BF3•O(C2H5)2 + 3 C6H5MgBr → B(C6H5)3 + 3 MgBrF + (C2H5)2O. It can also be synthesized on a smaller scale by the thermal decomposition of trimethylammonium tetraphenylborate. The equation is as follows: [B(C6H5)4][NH(CH3)3] → B(C6H5)3 + N(CH3)3 + C6H6.
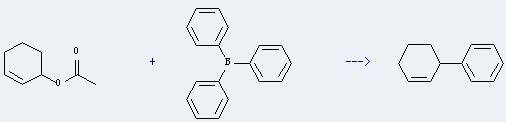
Uses of Triphenylborane: it can be used to make triarylborane amine complexes, such as pyridine-triphenylborane. And it can react with acetic acid cyclohex-2-enyl ester to get 3-phenyl-cyclohexene. This reaction will need catalysts palladium bis(dibenzylideneacetone) and PPh3, and solvent tetrahydrofuran. The reaction time is 12 hours at reaction temperature of 60 °C. The yield is about 52%.
When you are using this chemical, please be cautious about it as the following:
This chemical is highly flammable and harmful if swallowed. It may form explosive peroxides and irritating to eyes, respiratory system and skin. During using it, wear suitable protective clothing and avoid contact with skin and eyes. And you should not breathe dust. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. In addition, you should take precautionary measures against static discharges. Keep away from sources of ignition and no smoking at last.
You can still convert the following datas into molecular structure:
(1)SMILES: c3c(B(c1ccccc1)c2ccccc2)cccc3
(2)InChI: InChI=1/C18H15B/c1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18/h1-15H
(3)InChIKey: MXSVLWZRHLXFKH-UHFFFAOYAV
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View