- Min.Order :1 Kilogram
- Purity: 98%
- Payment Terms : T/T,
Keywords
1501-84-4 1-(1-adamantyl)ethanamine hydrochloride adamantane
Quick Details
- Appearance:white crystalline solid
- Application:1501-84-4 Uses of Rimantadine hydrochloride: this product is antiviral drug used for the treatment of influenza virus infection. Moreover, it can react with Acetyl chloride to get N-(1-Adamantan-1-yl
- PackAge:25kg/drum
- ProductionCapacity:10|Metric Ton|Month
- Storage:AMB
- Transportation:1501-84-4
Superiority:
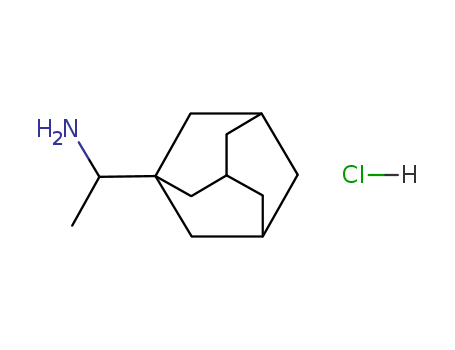
The IUPAC name of Rimantadine hydrochloride is 1-(1-adamantyl)ethanamine hydrochloride. With the CAS registry number 1501-84-4, it is also named as Adamantane, 1-(1-aminoethyl)-, hydrochloride. The product's categories are Adamantane Derivatives; Anti-viral Compounds; Adamantanes; Anti-virals; Inhibitors; Intermediates & Fine Chemicals; Non-nucleoside Reverse Transcriptase; Pharmaceuticals. Besides, it is white crystalline solid, which should be stored in tightly sealed containers in a cool, dry place. And you should ensure that the workplaces have good ventilation or exhaust devices. In addition, its molecular formula is C12H22ClN and molecular weight is 215.77.
The other characteristics of this product can be summarized as: (1)ACD/LogP: 3.10; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0; (4)ACD/LogD (pH 7.4): 0.08; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 1; (8)ACD/KOC (pH 7.4): 1.11; (9)#H bond acceptors: 1; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 3.24 Å2; (13)Flash Point: 99.3 °C; (14)Melting Point: 300 °C; (15)Enthalpy of Vaporization: 48.5 kJ/mol; (16)Boiling Point: 247.8 °C at 760 mmHg; (17)Vapour Pressure: 0.0251 mmHg at 25 °C.
Preparation of Rimantadine hydrochloride: frist of all, you can use Adamantane bromide to react with Vinyl bromide by catalyzing of Aluminium tribromide. And then use the resultant to react with Potassium hydroxide by heating. Afterwards, please use this resultant to react with Sulfuric acid, then react with hydroxylamine. At last, you would obtain this chemical by Lithium aluminium hydride reduction.
.gif)
Uses of Rimantadine hydrochloride: this product is antiviral drug used for the treatment of influenza virus infection. Moreover, it can react with Acetyl chloride to get N-(1-Adamantan-1-yl-ethyl)-acetamide.
.jpg)
This reaction needs KOH, Diethyl ether and H2O for 15 min. The yield is 95 %.
When you are using this chemical, please be cautious about it as the following: it is irritating to eyes, respiratory system and skin. It is also harmful if swallowed. In case of contact with eyes, please rinse immediately with plenty of water and seek medical advice. And you should wear suitable protective clothing.
People can use the following data to convert to the molecule structure.
(1)Canonical SMILES: CC(C12CC3CC(C1)CC(C3)C2)N.Cl
(2)InChI: InChI=1S/C12H21N.ClH/c1-8(13)12-5-9-2-10(6-12)4-11(3-9)7-12;/h8-11H,2-7,13H2,1H3;1H
(3)InChIKey: OZBDFBJXRJWNAV-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 135mg/kg (135mg/kg) |
BEHAVIORAL: MUSCLE WEAKNESS BEHAVIORAL: ATAXIA |
Pharmaceutical Chemistry Journal Vol. 11, Pg. 798, 1977. |
| rat | LD50 | oral | 640mg/kg (640mg/kg) | Voprosy Onkologii. Problems of Oncology. For English translation, see PONCAU. Vol. 28(9), Pg. 23, 1982 |
Details:
The IUPAC name of Rimantadine hydrochloride is 1-(1-adamantyl)ethanamine hydrochloride. With the CAS registry number 1501-84-4, it is also named as Adamantane, 1-(1-aminoethyl)-, hydrochloride. The product's categories are Adamantane Derivatives; Anti-viral Compounds; Adamantanes; Anti-virals; Inhibitors; Intermediates & Fine Chemicals; Non-nucleoside Reverse Transcriptase; Pharmaceuticals. Besides, it is white crystalline solid, which should be stored in tightly sealed containers in a cool, dry place. And you should ensure that the workplaces have good ventilation or exhaust devices. In addition, its molecular formula is C12H22ClN and molecular weight is 215.77.
The other characteristics of this product can be summarized as: (1)ACD/LogP: 3.10; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0; (4)ACD/LogD (pH 7.4): 0.08; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 1; (8)ACD/KOC (pH 7.4): 1.11; (9)#H bond acceptors: 1; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 3.24 Å2; (13)Flash Point: 99.3 °C; (14)Melting Point: 300 °C; (15)Enthalpy of Vaporization: 48.5 kJ/mol; (16)Boiling Point: 247.8 °C at 760 mmHg; (17)Vapour Pressure: 0.0251 mmHg at 25 °C.
Preparation of Rimantadine hydrochloride: frist of all, you can use Adamantane bromide to react with Vinyl bromide by catalyzing of Aluminium tribromide. And then use the resultant to react with Potassium hydroxide by heating. Afterwards, please use this resultant to react with Sulfuric acid, then react with hydroxylamine. At last, you would obtain this chemical by Lithium aluminium hydride reduction.
.gif)
Uses of Rimantadine hydrochloride: this product is antiviral drug used for the treatment of influenza virus infection. Moreover, it can react with Acetyl chloride to get N-(1-Adamantan-1-yl-ethyl)-acetamide.
.jpg)
This reaction needs KOH, Diethyl ether and H2O for 15 min. The yield is 95 %.
When you are using this chemical, please be cautious about it as the following: it is irritating to eyes, respiratory system and skin. It is also harmful if swallowed. In case of contact with eyes, please rinse immediately with plenty of water and seek medical advice. And you should wear suitable protective clothing.
People can use the following data to convert to the molecule structure.
(1)Canonical SMILES: CC(C12CC3CC(C1)CC(C3)C2)N.Cl
(2)InChI: InChI=1S/C12H21N.ClH/c1-8(13)12-5-9-2-10(6-12)4-11(3-9)7-12;/h8-11H,2-7,13H2,1H3;1H
(3)InChIKey: OZBDFBJXRJWNAV-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 135mg/kg (135mg/kg) |
BEHAVIORAL: MUSCLE WEAKNESS BEHAVIORAL: ATAXIA |
Pharmaceutical Chemistry Journal Vol. 11, Pg. 798, 1977. |
| rat | LD50 | oral | 640mg/kg (640mg/kg) | Voprosy Onkologii. Problems of Oncology. For English translation, see PONCAU. Vol. 28(9), Pg. 23, 1982 |
You Might Also Like
Related Searches
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View