-
Name
(2-Methylphenoxy)acetic acid
- EINECS 217-517-4
- CAS No. 1878-49-5
- Article Data62
- CAS DataBase
- Density 1.179 g/cm3
- Solubility
- Melting Point 155-157 °C(lit.)
- Formula C9H10O3
- Boiling Point 288.4 °C at 760 mmHg
- Molecular Weight 166.177
- Flash Point 115.7 °C
- Transport Information
- Appearance white needle-like crystal
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Aceticacid, (2-methylphenoxy)- (9CI);Acetic acid, (o-tolyloxy)- (6CI,7CI,8CI);(o-Methylphenoxy)acetic acid;2-(2-Methylphenoxy)acetic acid;2-(o-Tolyloxy)acetic acid;2-[(2-Methylphenyl)oxy]acetic acid;NSC 5293;o-Cresolglycolic acid;o-Cresoxyacetic acid;o-Cresyloxyacetic acid;o-Tolyloxyacetic acid;
- PSA 46.53000
- LogP 1.45840
Synthetic route
-
-
7748-25-6
potassium chloroacetate

-
-
3235-09-4
potassium o-cresolate

-
-
1878-49-5
o-methylphenoxyacetic acid

| Conditions | Yield |
|---|---|
| Stage #1: potassium chloroacetate; potassium o-cresolate In water for 2h; Reflux; Stage #2: With hydrogenchloride In water at 30℃; pH=1; | 98% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; bentonite In water for 0.0833333h; microwave irradiation; | 94% |
| With sodium hydroxide In water at 100℃; for 2h; Inert atmosphere; | 51% |
| With sodium hydroxide In water Reflux; | 15% |
-
-
2989-17-5
methyl 2-methylphenoxy acetate

-
A
-
1878-49-5
o-methylphenoxyacetic acid

-
B
-
95-48-7
ortho-cresol

| Conditions | Yield |
|---|---|
| With lithium chloride In N,N-dimethyl-formamide for 22h; Heating; | A 90% B 5% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water for 1h; Reflux; | 89.3% |
| With sodium hydroxide at 120℃; pH=8 - 9; pH-value; |
-
-
93917-68-1
ethyl (2-methylphenoxy)acetate

-
-
1878-49-5
o-methylphenoxyacetic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol Reflux; | 86% |
| With sodium hydroxide In ethanol; water Reflux; | 81% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; caesium carbonate In water; dimethyl sulfoxide at 120℃; for 24h; Inert atmosphere; Schlenk technique; | 82% |

| Conditions | Yield |
|---|---|
| With butan-1-ol anschliessend Erhitzen mit wss. NaOH; |
-
-
22560-43-6
ortho-methylphenoxyacetamide

-
-
1878-49-5
o-methylphenoxyacetic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide |

| Conditions | Yield |
|---|---|
| With ethanol Erwaermen des Reaktionsgemisches mit wss. NaOH; |

| Conditions | Yield |
|---|---|
| In ethyl acetate |

-
-
1878-49-5
o-methylphenoxyacetic acid

| Conditions | Yield |
|---|---|
| With air moisture |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol | |
| With sodium hydroxide In water for 0.05h; microwave irradiation; | |
| With water; sodium hydroxide Microwave irradiation; | |
| With lithium hydroxide In methanol; water at 50℃; for 12h; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: K2CO3; KI; PEG-600 / dimethylformamide / 0.07 h / microwave irradiation 2: NaOH / H2O / 0.05 h / microwave irradiation View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NaH 2: aq. LiOH / tetrahydrofuran View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: K2CO3 / dimethylformamide 2: aq. NaOH / methanol View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 160 °C View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: MCPA With naphthalene radical anion sodium salt In tetrahydrofuran at 0 - 20℃; for 12h; Inert atmosphere; Stage #2: With hydrogenchloride In tetrahydrofuran; water chemoselective reaction; | |
| With 0.5% Pd/Sibunit carbon material; hydrogen In water at 30℃; under 760.051 Torr; for 100h; | |
| With hydrogen In water at 30℃; under 760.051 Torr; | |
| With palladium/alumina; titanium(IV) oxide for 0.5h; Irradiation; |

| Conditions | Yield |
|---|---|
| Stage #1: ethyl bromoacetate; ortho-cresol With potassium carbonate In acetonitrile at 80℃; for 4h; Stage #2: With water; sodium hydroxide In ethanol at 50℃; for 4h; | |
| Stage #1: ethyl bromoacetate; ortho-cresol With potassium carbonate In dimethyl sulfoxide at 50℃; for 6h; Stage #2: With water; sodium hydroxide In acetone at 50℃; for 3h; Stage #3: With hydrogenchloride In water at 25℃; pH=1 - 2; | |
| Stage #1: ethyl bromoacetate; ortho-cresol With potassium carbonate In acetonitrile at 80℃; for 4h; Stage #2: With water; sodium hydroxide In ethanol at 50℃; for 4h; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium iodide; potassium carbonate / N,N-dimethyl-formamide / 12 h / 75 °C 2: potassium hydroxide / ethanol; water / 1.5 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sodium hydroxide / water / 8 h / Reflux 2: hydrogenchloride / water / pH 1 View Scheme | |
| Multi-step reaction with 2 steps 1: potassium carbonate / acetonitrile / 5 h / Reflux 2: water; sodium hydroxide / ethanol / 4 h / 20 °C View Scheme |
-
-
57548-58-0
Sodium; o-tolyloxy-acetate

-
-
1878-49-5
o-methylphenoxyacetic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water pH=1; |

-
A
-
1878-49-5
o-methylphenoxyacetic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium tert-butylate / tetrahydrofuran / 24 h / 20 °C 2: glucose dehydrogenase; D-glucose; SyADH from sphingobium yanoikuyae; nicotinamide adenine dinucleotide phosphate / aq. acetate buffer / 1 h / 30 °C / pH 5 / Enzymatic reaction View Scheme |


-
A
-
1878-49-5
o-methylphenoxyacetic acid

| Conditions | Yield |
|---|---|
| With glucose dehydrogenase; D-glucose; SyADH from sphingobium yanoikuyae; nicotinamide adenine dinucleotide phosphate In aq. acetate buffer at 30℃; for 1h; pH=5; Enzymatic reaction; stereoselective reaction; | A n/a B n/a C n/a |

-
-
1878-49-5
o-methylphenoxyacetic acid

-
-
15516-43-5
(2-methylphenoxy)acetyl chloride

| Conditions | Yield |
|---|---|
| With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane | 100% |
| With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane at 20℃; for 1h; | 100% |
| With thionyl chloride In benzene for 3h; Reflux; | 92% |
-
-
1878-49-5
o-methylphenoxyacetic acid

-
-
637-39-8
triethanolamine hydrochloride

-
-
55543-68-5
2-(2-methylphenyloxy)acetate of tris(2-hydroxyethyl)-ammonium

| Conditions | Yield |
|---|---|
| Stage #1: o-methylphenoxyacetic acid for 1h; Reflux; Alkaline conditions; Stage #2: triethanolamine hydrochloride Reflux; | 99.8% |
| Stage #1: o-methylphenoxyacetic acid for 1h; Alkaline conditions; Reflux; Stage #2: triethanolamine hydrochloride Alkaline conditions; | 99.8% |
-
-
1878-49-5
o-methylphenoxyacetic acid

-
-
713517-90-9
1-(2,6-dichlorobenzyl)-5-phenyl-1,3-dihydrobenzo[e][1,4]diazepin-2-one

| Conditions | Yield |
|---|---|
| With bis-(2-oxo-3-oxazolidinyl)phosphoryl chloride; triethylamine In dichloromethane at 0 - 20℃; | 99% |
-
-
1878-49-5
o-methylphenoxyacetic acid

| Conditions | Yield |
|---|---|
| With calcium oxide In benzene Reflux; | 99% |

| Conditions | Yield |
|---|---|
| With sulfuryl dichloride; aluminium In diethyl ether for 4h; Reflux; | 98% |
| With dihydrogen peroxide; chlorine In chlorobenzene at 75℃; | 98% |
| With chlorine In toluene at 50 - 55℃; for 2h; Temperature; | 76.8% |
-
-
1878-49-5
o-methylphenoxyacetic acid

-
-
23978-55-4
1,4,10,13-tetraoxa-7,16-diazacyclooctadecane

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 12h; | 98% |

| Conditions | Yield |
|---|---|
| In benzene soln. of aroxyacetic acid in benzene contg. ZnO refluxed under stirring for 8 h; evapn. under vac., residue washed with ether, dried under vac.; elem. anal.; | 97% |

| Conditions | Yield |
|---|---|
| In benzene prepn. by refluxing mixt. of 2-methylphenoxyacetic acid and ZnO in C6H6 for 8 h; elem. anal.; | 97% |
| In not given | 97% |
-
-
1878-49-5
o-methylphenoxyacetic acid

-
-
57-14-7
1,1-dimethylhydrazine

-
-
1401314-34-8
1,1-dimethylhydrazinium 2-(2-methylphenoxy)acetate

| Conditions | Yield |
|---|---|
| at 45℃; for 0.25h; | 97% |
-
-
42974-19-6
benzo[b]furan-2-carbohydrazide

-
-
1878-49-5
o-methylphenoxyacetic acid

-
-
1019843-41-4
C18H14N2O3

| Conditions | Yield |
|---|---|
| With pyridine; trichlorophosphate for 8h; Reflux; | 96% |
-
-
1878-49-5
o-methylphenoxyacetic acid

-
-
1448165-42-1
N-(3-silatranylpropyl)ammonium-2-methylphenyloxyacetate

| Conditions | Yield |
|---|---|
| In methanol at 15℃; for 0.166667h; | 96% |

| Conditions | Yield |
|---|---|
| With sulfuric acid for 0.0333333h; microwave irradiation; | 95% |
| acid at 30℃; Rate constant; other temperature: 40 deg C; | |
| With hydrogenchloride | |
| With sulfuric acid for 3h; Heating; |
-
-
101182-23-4
1-hydroxygermatrane monohydrate

-
-
1878-49-5
o-methylphenoxyacetic acid

-
-
1460243-62-2
C15H21GeNO6

| Conditions | Yield |
|---|---|
| In 5,5-dimethyl-1,3-cyclohexadiene for 4h; Heating; Dean-Stark; | 94.3% |
-
-
10489-99-3
N,N-dimethyl-mono(2-hydroxyethyl)amine-N-oxide

-
-
1878-49-5
o-methylphenoxyacetic acid

| Conditions | Yield |
|---|---|
| In methanol at 25℃; for 1h; | 94% |
-
-
18364-47-1
N-methyl-N-(3-pyridyl)amine

-
-
1878-49-5
o-methylphenoxyacetic acid

| Conditions | Yield |
|---|---|
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 16h; | 93% |
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 16h; | 43% |
-
-
1878-49-5
o-methylphenoxyacetic acid


| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In N,N-dimethyl-formamide at 0 - 20℃; | 92% |
-
-
1878-49-5
o-methylphenoxyacetic acid

-
-
12775-96-1, 15158-11-9, 15721-63-8, 16941-75-6, 17493-86-6, 19498-52-3, 20499-83-6, 20499-84-7, 20499-85-8, 20499-86-9, 20573-10-8, 20573-11-9, 21595-51-7, 21595-52-8, 22206-52-6, 26445-28-3, 28959-95-7, 37362-93-9, 39417-05-5, 54603-16-6, 54603-23-5, 54603-32-6, 54603-40-6, 54603-48-4, 54603-81-5, 54603-89-3, 56316-56-4, 95985-91-4, 122297-32-9, 7440-50-8
copper

| Conditions | Yield |
|---|---|
| In benzene soln. of aroxyacetic acid in benzene contg. activated Cu refluxed under stirring for 12 h; evapn. under vac., residue washed with ether, dried under vac.; elem. anal.; | 92% |

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid In dichloromethane at 0 - 20℃; Inert atmosphere; | 92% |

| Conditions | Yield |
|---|---|
| In methanol at 65℃; | 92% |
-
-
1878-49-5
o-methylphenoxyacetic acid

-
-
87269-87-2, 87679-25-2, 88547-79-9, 93779-29-4, 94569-19-4, 138877-09-5
benzyl (2S,3AS,6AS)-octahydrocyclopenta[b]pyrrole-2-carboxylate hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: o-methylphenoxyacetic acid; benzyl (2S,3AS,6AS)-octahydrocyclopenta[b]pyrrole-2-carboxylate hydrochloride With benzotriazol-1-ol; triethylamine In dichloromethane at 0 - 25℃; for 0.25h; Inert atmosphere; Stage #2: With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 20℃; for 12.5h; Inert atmosphere; | 92% |
-
-
1878-49-5
o-methylphenoxyacetic acid

-
-
147318-83-0
[(1S,2S)-1-Benzyl-3-((R)-4-tert-butylcarbamoyl-thiazolidin-3-yl)-2-hydroxy-3-oxo-propyl]-carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; | 91% |

-
-
1878-49-5
o-methylphenoxyacetic acid

| Conditions | Yield |
|---|---|
| In methanol soln. of Zn compd. and aroxyacetic acid in MeOH refluxed for 12 h; evapn. under vac., crystd. from THF; | 90% |

| Conditions | Yield |
|---|---|
| 89% | |
| 89% | |
| With lithium aluminium tetrahydride; diethyl ether |

| Conditions | Yield |
|---|---|
| In methanol at 45℃; for 3h; | 89% |

| Conditions | Yield |
|---|---|
| With triethylamine; 2-hydroxy-2-methylpropanenitrile; 1,1'-carbonyldiimidazole In dichloromethane at 25℃; for 12h; | 88% |
(2-Methylphenoxy)acetic acid Specification
The Acetic acid,2-(2-methylphenoxy)- is an organic compound with the formula C9H10O3. The IUPAC name of this chemical is 2-(2-methylphenoxy)acetic acid. With the CAS registry number 1878-49-5, it is also named as 2-(2-methylphenoxy)acetic acid. The product's categories are Phenoxyacetic Acids and Alcohols (substituted); C9; Carbonyl Compounds; Carboxylic Acids. Besides, it is a white needle-like crystal, which should be stored in a closed cool and dry place.
Physical properties about Acetic acid,2-(2-methylphenoxy)- are: (1)ACD/LogP: 1.80; (2)ACD/LogD (pH 5.5): -0.46; (3)ACD/LogD (pH 7.4): -1.81; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 1.24; (7)ACD/KOC (pH 7.4): 1; (8)#H bond acceptors: 3; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 3; (11)Polar Surface Area: 35.53 Å2; (12)Index of Refraction: 1.536; (13)Molar Refractivity: 43.95 cm3; (14)Molar Volume: 140.8 cm3; (15)Polarizability: 17.42×10-24cm3; (16)Surface Tension: 44.2 dyne/cm; (17)Density: 1.179 g/cm3; (18)Flash Point: 115.7 °C; (19)Enthalpy of Vaporization: 55.73 kJ/mol; (20)Boiling Point: 288.4 °C at 760 mmHg; (21)Vapour Pressure: 0.00109 mmHg at 25°C.
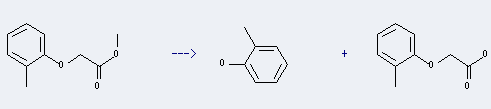
Preparation: this chemical can be prepared by o-tolyloxy-acetic acid methyl ester. This reaction will need reagent LiCl and solvent dimethylformamide. The reaction time is 22 hours by heating. The yield is about 90%.
Uses of Acetic acid,2-(2-methylphenoxy)-: it can be used to produce 4-amino-5-o-tolyloxymethyl-2,4-dihydro-[1,2,4]triazole-3-thione at temperature of 180 °C. The reaction time is 15 min. The yield is about 72%.

When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. When you are using it, wear suitable protective clothing. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(O)COc1ccccc1C
(2)InChI: InChI=1/C9H10O3/c1-7-4-2-3-5-8(7)12-6-9(10)11/h2-5H,6H2,1H3,(H,10,11)
(3)InChIKey: QJVXBRUGKLCUMY-UHFFFAOYAX
(4)Std. InChI: InChI=1S/C9H10O3/c1-7-4-2-3-5-8(7)12-6-9(10)11/h2-5H,6H2,1H3,(H,10,11)
(5)Std. InChIKey: QJVXBRUGKLCUMY-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | intraperitoneal | 452mg/kg (452mg/kg) | Travaux de la Societe de Pharmacie de Montpellier. Vol. 34, Pg. 121, 1974. |
Related Products
- (2-Methylphenoxy)acetic acid
- 18786-24-8
- 187863-42-9
- 187865-22-1
- 1878-65-5
- 1878-66-6
- 1878-67-7
- 1878-68-8
- 1878-69-9
- 18787-63-8
- 1878-80-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View