-
Name
3-HYDROXY-1,2-DIMETHYL-4(1H)-PYRIDONE
- EINECS 212-783-8
- CAS No. 30652-11-0
- Article Data22
- CAS DataBase
- Density 1.225 g/cm3
- Solubility soluble in hot water
- Melting Point 272-275 °C(lit.)
- Formula C7H9NO2
- Boiling Point 232.7 °C at 760 mmHg
- Molecular Weight 139.154
- Flash Point 94.5 °C
- Transport Information
- Appearance White needles
- Safety 26-36-37/39
- Risk Codes 22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 4(1H)-Pyridone,3-hydroxy-1,2-dimethyl- (8CI);1,2-Dimethyl-3-hydroxy-4(1H)-pyridinone;1,2-Dimethyl-3-hydroxypyridin-4-one;1,2-Dimethyl-3-hydroxypyridine-4-one;3-Hydroxy-1,2-dimethyl-4(1H)-pyridinone;3-Hydroxy-1,2-dimethyl-4(1H)-pyridone;3-Hydroxy-1,2-dimethyl-4-pyridinone;3-Hydroxy-1,2-dimethyl-4-pyridone;APO 66;CGP 37391;CP 20 (chelatingagent);DN 18001AF;Deferiprone;Ferriprox;L 1 (chelatingagent);
- PSA 42.23000
- LogP 0.39930
Synthetic route
-
-
90037-22-2
1,2-dimethyl-3-(benzyloxy)-4(1H)-pyridinone

-
-
30652-11-0
deferiprone

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol for 2h; | 80% |
| With hydrogen; palladium on activated charcoal In tetrahydrofuran under ambient conditions; | 65% |
| With concentrated hydrobromic acid |

| Conditions | Yield |
|---|---|
| In water for 10h; Reflux; | 69% |
| In water at 160 - 162℃; under 6750.5 Torr; for 0.0216667h; Irradiation; | 65% |
| In water for 6.5h; Heating; | 60% |

| Conditions | Yield |
|---|---|
| In water for 20h; Heating; | 64% |
-
-
82756-28-3
3-(β-D-galactopyranosyloxy)-2-furanyl methyl ketone

-
-
74-89-5
methylamine

-
-
30652-11-0
deferiprone

| Conditions | Yield |
|---|---|
| In water for 20h; Heating; | 49% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water at 50℃; | 25% |
-
-
30652-11-0
deferiprone

| Conditions | Yield |
|---|---|
| With (C2H5)3N In methanol; dichloromethane (C2H5)3N added to a soln. of Co complex, a soln. of ligand in MeOH added, stirred at room temp. overnight under N2; evapd. (vac.), dissolved in C6H6, filtered, crystd. by diffusion of the soln. with pentane; elem. anal.; | 98% |
-
-
30652-11-0
deferiprone

-
-
100-21-0
terephthalic acid

-
-
7732-18-5
water

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

| Conditions | Yield |
|---|---|
| at 100℃; for 72h; Sealed tube; High pressure; | 98% |


| Conditions | Yield |
|---|---|
| In methanol; water addn. of soln. of tantalum compd. in methanol to soln. of deferiprone inmethanol, stirring at room temp. overnight, addn. of water, stirring fo r 30 min; evapn. at 40°C, washing with warm CHCl3, filtration, drying in vac., elem. anal.; | 97% |


-
-
30652-11-0
deferiprone

-
-
176792-83-9
[RuCl(C7H8NO2)(C6H3(CH3)3)]

| Conditions | Yield |
|---|---|
| With NaOCH3 In methanol; water byproducts: NaCl; (N2); reflux (3 h); solvent removal, dissoln. (CH2Cl2), filtration (Celite), evapn.; elem. anal.; | 93% |
-
-
30652-11-0
deferiprone

-
-
333-27-7
methyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| In chloroform for 3h; Heating; | 90% |

| Conditions | Yield |
|---|---|
| In methanol byproducts: NaCl; N2-atmosphere; stoich. amts., refluxing for 3 h; solvent removal, extn. into CH2Cl2, filtration (Celite), evapn., recrystn. (CH2Cl2/Et2O); | 90% |

-
-
30652-11-0
deferiprone

-
-
7646-85-7
zinc(II) chloride

| Conditions | Yield |
|---|---|
| With NaOH In methanol; water raising of the pH to 7 with dilute NaOH, concn.,; cooling, filtration, elem. anal.; | 90% |

| Conditions | Yield |
|---|---|
| In ethanol; acetone at 60℃; for 2h; | 90% |
| In ethanol; acetone at 20 - 45℃; for 73.7h; Sealed tube; | 36.5 mg |

| Conditions | Yield |
|---|---|
| In water at 60℃; for 2h; | 90% |
-
-
30652-11-0
deferiprone

-
-
20644-97-7
vanadyl

| Conditions | Yield |
|---|---|
| With NaOH In water under Ar; ligand suspended in water; heated at 50-60°C; VO(2+) soln. and solid NaOH added; chilled; filtered; solid washed with water and acetone; dried in vac.; elem. anal.; | 86% |
-
-
30652-11-0
deferiprone

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
1259401-68-7
1,2-dimethyl-4-oxo-1,4-dihydropyridin-3-yl 4-methylbenzenesulfonate

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; Inert atmosphere; | 86% |

-
-
30652-11-0
deferiprone

-
-
173413-67-7
Rh(C5(CH3)5)Cl(C5H2NO2(CH3)2)

| Conditions | Yield |
|---|---|
| 85% |
-
-
30652-11-0
deferiprone

-
-
52462-29-0
[ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2

-
-
252573-94-7
[(p-cymene)Ru(C7H8NO2)Cl]

| Conditions | Yield |
|---|---|
| With NaOMe In methanol; dichloromethane addn. of 1 equiv. NaOMe (in MeOH) to ligand (in CH2Cl2), addn. to stoich. amt. of metal complex (in CH2Cl2), standing for 2 h; solvent removal (vac.), extn. into Et2O/CH2Cl2=1:2, evapn. (reduced pressur), drying (vac.); elem. anal.; | 85% |

-
-
30652-11-0
deferiprone

| Conditions | Yield |
|---|---|
| With MeONa In methanol; dichloromethane inert atm.; 4 h; evapn. (vac.), extraction (Et2O/CH2Cl2 1:2), evapn., drying (vac.); elem. anal.; | 85% |
-
-
30652-11-0
deferiprone

-
-
6156-78-1
manganese (II) acetate tetrahydrate

| Conditions | Yield |
|---|---|
| With sodium acetate trihydrate In methanol to soln. ligand and AcONa*3H2O in MeOH Mn(OAc)2*4H2O was added and refluxed for 4 h; react. mixt. was cooled to room temp., ppt. was filtered, washed with Et2O and dried in vacuo overnight; elem. anal.; | 84.6% |

| Conditions | Yield |
|---|---|
| With triethylamine In methanol CrCl3*6H2O added to soln. of deferiprone and triethylamine in CH3OH, mixt. refluxed for 4 h, cooled to room temp.; solid filtered off, washed with cold methanol and ether, dried under vac., recrystd. from water; elem. anal.; | 84.4% |
-
-
30652-11-0
deferiprone

-
-
186321-59-5, 175662-51-8
bis(1,4-dihydro-1,2-dimethyl-4-oxo-3-pyridinolato)oxovanadium (IV)

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water to a soln. of 1-hydroxy-4,6-dimethyl-2(1H)-pyrimidinone in water added dropwise a soln. of VOSO4*3.6H2O in water, pH of the soln. adjusted to 10with 2M KOH, stirred overnight; precipitate collected by filtration, washed several times with H2O, dried over anhydrous P2O5 in vacuo, elem. anal.; | 84% |
-
-
30652-11-0
deferiprone

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile to soln. ligand and Et3N in MeCN MnCl2 was added and refluxed for 6 h; react. mixt. was cooled to room temp. and concd. in vacuo, cold Et2O wasadded, ppt. was filtered, washed with Et2O and dried in vacuo overnight ; elem. anal.; | 83.8% |

| Conditions | Yield |
|---|---|
| With silver(l) oxide at 45℃; for 2h; | 83% |

-
-
30652-11-0
deferiprone

| Conditions | Yield |
|---|---|
| With KOH In water under aerobic conditions; VOSO4*3H2O in hot water added slowly to suspn.of ligand (molar ratio 1:2) in hot water; aq. soln. of KOH (2 equiv.) a dded (pH ca. 3); refluxed for 2 h; ppt. collected; washed with acetone; dried overnight in vac.; satisfactory elem. anal.; | 83% |
-
-
30652-11-0
deferiprone

-
-
250638-50-7
[Rh(CO)2(CH3NCHCHCOCOC(CH3))]

| Conditions | Yield |
|---|---|
| With MeONa In methanol; dichloromethane 4 h; evapn. (vac.), extraction (Et2O/CH2Cl2 1:2), evapn., drying (vac.); elem. anal.; | 83% |
-
-
30652-11-0
deferiprone

-
-
250717-07-8, 250638-60-9
[Pd(CH3NCHCHCOCOC(CH3))((CH3)2NCH2C6H4)]

| Conditions | Yield |
|---|---|
| With NaOMe In methanol; dichloromethane 2 h; evapn. (vac.), extraction (Et2O/CH2Cl2), evapn., drying (vac., 50°C); elem. anal.; | 83% |
-
-
30652-11-0
deferiprone

-
-
7732-18-5
water

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile under N2 atm. to slurry MnCl2 in MeCN was added soln. ligand and Et3N inMeCN, mixt. was refluxed for overnight; soln. was cooled to room temp., volume was reduced by stream N2 gas, cold Et2O was added, solid was filtered, washed with MeCN, Et2O, dried under vac. overnight; elem. anal.; | 83% |


| Conditions | Yield |
|---|---|
| In ethanol at 95℃; for 24h; | 82% |


| Conditions | Yield |
|---|---|
| With NH3 In water 3-hydroxy-1,2-dimethyl-4-pyridinone was added to BeSO4*4H2O in water, after dissolution pH was adjusted to 8.5 with concd. NH3; solvent was evapd. at 323-333 K and cooled to room temp., ppt. was filtered off, washed with cold water, and dried in air, recrystn. from MeOH/Et2O; elem. anal.; | 81% |

-
-
30652-11-0
deferiprone

| Conditions | Yield |
|---|---|
| With NaOMe In methanol; dichloromethane 2 h; evapn. (vac.), extraction (Et2O/CH2Cl2), evapn., drying (vac., 50°C); elem. anal.; | 80% |
-
-
30652-11-0
deferiprone

| Conditions | Yield |
|---|---|
| In water slow evapn. of aq. soln. of 0.1 M NaClO4, pyridone-compound, uranyl-compound at room temp. at pH 3; elem. anal.; | 80% |

-
-
30652-11-0
deferiprone

-
-
250638-59-6
[PdCl(CH3NCHCHCOCOC(CH3))(P(C4H9)3)]

| Conditions | Yield |
|---|---|
| With NaOMe In methanol; dichloromethane inert atm.; 2 h; evapn. (vac.), extraction (Et2O/CH2Cl2), evapn., drying (vac., 50°C); elem. anal.; | 78% |

| Conditions | Yield |
|---|---|
| at 100℃; for 24h; | 77% |

1,2-Dimethyl-3-hydroxy-4-pyridone Chemical Properties
Chemical Name: 3-Hydroxy-1,2-dimethyl-4(1H)-pyridone
IUPAC NAME: 3-Hydroxy-1,2-dimethylpyridin-4-one
CAS No.: 30652-11-0
RTECS: UU7785940
Molecular Formula: C7H9NO2
Molecular Weight: 139.15 g/mol
Melting Point: 272-275 °C(lit.)
Density: 1.225 g/cm3
Flash Point: 94.5 °C
Boiling Point: 232.7 °C at 760 mmHg
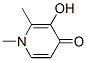
Following is the structure of 1,2-Dimethyl-3-hydroxy-4-pyridone (30652-11-0):
Product Categories about 1,2-Dimethyl-3-hydroxy-4-pyridone (30652-11-0) are API intermediates ; Chelating Agents, Ligands ; Chelating Agents & Ligands ; Intermediates & Fine Chemicals ; Pharmaceuticals ; Building Blocks ; Heterocyclic Building Blocks ; Piperidones
The chemical synonymous of 1,2-Dimethyl-3-hydroxy-4-pyridone (30652-11-0) are L1 ; Dmhp ; Deferidone ; Deferipron ; Deferiprone ; Cp20 ; 3-Hydroxy-1,2-Dimethyl-4(1h)Pyridinone ; 3-Hydroxy-1,2-Dimethyl-4(1h)-Pyridone
1,2-Dimethyl-3-hydroxy-4-pyridone Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LDLo | oral | 1gm/kg (1000mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: FOOD INTAKE (ANIMAL) GASTROINTESTINAL: ULCERATION OR BLEEDING FROM STOMACH | BioMetals. Vol. 10, Pg. 247, 1997. |
| mammal (species unspecified) | LD50 | unreported | > 1gm/kg (1000mg/kg) | Archives of Toxicology, Supplement. Vol. 18, Pg. 202, 1996. | |
| mouse | LD50 | intraperitoneal | 983mg/kg (983mg/kg) | Blood. Vol. 76, Pg. 2389, 1990. | |
| rat | LD50 | intraperitoneal | 600mg/kg (600mg/kg) | Drugs of the Future. Vol. 13, Pg. 413, 1988. | |
| rat | LD50 | oral | 2gm/kg (2000mg/kg) | CARDIAC: OTHER CHANGES | Archives of Toxicology, Supplement. Vol. 18, Pg. 202, 1996. |
1,2-Dimethyl-3-hydroxy-4-pyridone Safety Profile
Moderately toxic by ingestion and intraperitoneal routes. When heated to decomposition it emits toxic vapors of NOx.
Hazard Codes:
Xn: Harmful ![]()
Risk Statements about 1,2-Dimethyl-3-hydroxy-4-pyridone (30652-11-0):
R22 Harmful if swallowed.
R36/37/38 Irritating to eyes, respiratory system and skin.
Safety Statements about 1,2-Dimethyl-3-hydroxy-4-pyridone (30652-11-0):
S26 In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S37/39 Wear suitable gloves and eye/face protection.
Attentions:
1. Storage: Store in a cool, dry place. Store in a tightly closed container.
2. Handling: Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes. Avoid ingestion and inhalation.
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 30657-38-6
- 3065-79-0
- 306-60-5
- 3066-71-5
- 306-67-2
- 3066-75-9
- 3066-84-0
- 3066-86-2
- 30669-07-9
- 3066-90-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View