-
Name
2-Fluoro-4-nitrobenzoic acid
- EINECS -0
- CAS No. 403-24-7
- Article Data27
- CAS DataBase
- Density 1.568 g/cm3
- Solubility
- Melting Point 170 °C
- Formula C7H4FNO4
- Boiling Point 352.5 °C at 760 mmHg
- Molecular Weight 185.111
- Flash Point 167 °C
- Transport Information
- Appearance white to light yellow crystal powder
- Safety 26-37/39-36/37/39-22
- Risk Codes 36/37/38-22
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi,  Xn
Xn
- Synonyms NSC 190361;2-fluoro-4-nitrobenzenecarboxylic acid;
- PSA 83.12000
- LogP 1.95530
Synthetic route

| Conditions | Yield |
|---|---|
| With chromium(VI) oxide; periodic acid In acetonitrile for 1h; | 81% |
| With chromium(VI) oxide; periodic acid In acetonitrile for 1h; | 81% |
| With chromium(VI) oxide; periodic acid In acetonitrile for 1h; Inert atmosphere; | 81% |
-
-
866579-96-6
1-(2-fluoro-4-nitrophenyl)ethanone

-
-
403-24-7
2-fluoro-4-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With copper (II) nitrate tetrahydrate; oxygen In acetonitrile at 180℃; under 18751.9 Torr; for 1.5h; Green chemistry; | 69% |

| Conditions | Yield |
|---|---|
| With potassium dichromate; sulfuric acid at 90℃; for 3h; | 62% |

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethyl acetate at 20℃; under 760 Torr; | 100% |
| With hydrogen; palladium over charcoal In methanol; acetic acid at 20℃; under 760.051 Torr; | 100% |
| With hydrogen; palladium 10% on activated carbon In methanol | 100% |
| With ammonium hydroxide; iron(II) sulfate | |
| With palladium 10% on activated carbon; hydrogen In methanol at 20℃; for 16h; |

| Conditions | Yield |
|---|---|
| With thionyl chloride at 80℃; for 3h; | 100% |
| With thionyl chloride In toluene for 3h; Heating; | 86% |
| With thionyl chloride |
-
-
403-24-7
2-fluoro-4-nitrobenzoic acid

-
-
18107-18-1
diazomethyl-trimethyl-silane

-
-
392-09-6
2-fluoro-4-nitro-benzoic acid methyl ester

| Conditions | Yield |
|---|---|
| In methanol; diethyl ether; toluene at 20℃; for 1.5h; | 100% |
| In methanol |
-
-
403-24-7
2-fluoro-4-nitrobenzoic acid

-
-
123183-72-2
N-(tert-butyl)oxycarbonyl-N,N’-trimethyl-1,3-diaminopropane

-
-
1246246-94-5
tert-Butyl (3-{[(2-fluoro-4-nitrophenyl)carbonyl](methyl)amino}propyl)methylcarbamate

| Conditions | Yield |
|---|---|
| Stage #1: 2-fluoro-4-nitrobenzoic acid With pivaloyl chloride; triethylamine In dichloromethane at 20℃; for 0.25h; Cooling with ice; Stage #2: N-(tert-butyl)oxycarbonyl-N,N’-trimethyl-1,3-diaminopropane In dichloromethane at 20℃; for 2h; Cooling with ice; | 100% |
-
-
403-24-7
2-fluoro-4-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane at 23℃; | 100% |
-
-
67-56-1
methanol

-
-
403-24-7
2-fluoro-4-nitrobenzoic acid

-
-
392-09-6
2-fluoro-4-nitro-benzoic acid methyl ester

| Conditions | Yield |
|---|---|
| With sulfuric acid for 4h; Reflux; | 99% |
| With sulfuric acid Reflux; | 98% |
| With sulfuric acid for 15h; Reflux; Large scale; | 95% |
-
-
403-24-7
2-fluoro-4-nitrobenzoic acid

-
-
74-89-5
methylamine

-
-
49565-62-0
2-(methylamino)-4-nitro-benzoic acid

| Conditions | Yield |
|---|---|
| In ethanol at 80℃; for 16h; Sealed tube; | 91% |
-
-
403-24-7
2-fluoro-4-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2-fluoro-4-nitrobenzoic acid With thionyl chloride In dichloromethane for 2h; Reflux; Stage #2: ethyl 5,6-dihydro-4H-benzo[b]thieno[2,3-d]azepine-2-carboxylate hydrobromide With pyridine In acetonitrile at 20 - 50℃; for 5h; | 88% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-fluoro-4-nitrobenzoic acid With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane at 25℃; for 4h; Stage #2: With ammonia In water for 0.166667h; | 85% |
| With pyridine; ammonium chloride; 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide In ethyl acetate | 53% |
| With pyridine; ammonium chloride; 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide | 53% |
-
-
403-24-7
2-fluoro-4-nitrobenzoic acid

-
-
6638-79-5
N,O-dimethylhydroxylamine*hydrochloride

-
-
774239-17-7
2-fluoro-N-methoxy-N-methyl-4-nitrobenzamide

| Conditions | Yield |
|---|---|
| With pyridine; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride at 60℃; for 15h; | 85% |
| With pyridine; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride at 20℃; |
-
-
403-24-7
2-fluoro-4-nitrobenzoic acid

-
-
157701-72-9
4-nitro-2-fluorobenzaldehyde

| Conditions | Yield |
|---|---|
| With borane-THF In tetrahydrofuran at 80℃; for 3h; | 83% |
-
-
403-24-7
2-fluoro-4-nitrobenzoic acid

-
-
506-59-2
N,N-dimethylammonium chloride

-
-
1187368-66-6
2-fluoro-N,N-dimethyl-4-nitro-benzamide

| Conditions | Yield |
|---|---|
| With ethylene dichloride hydrochloride; benzotriazol-1-ol; triethylamine In N,N-dimethyl-formamide at 20℃; for 12h; | 81% |
| Stage #1: 2-fluoro-4-nitrobenzoic acid With 1,1'-carbonyldiimidazole In dichloromethane at 20℃; for 1h; Stage #2: N,N-dimethylammonium chloride With triethylamine In dichloromethane for 1h; | 60% |
-
-
41838-46-4
1-methyl-4-aminopiperidine

-
-
403-24-7
2-fluoro-4-nitrobenzoic acid

-
-
957855-56-0
2-fluoro-N-(1-methyl-4-piperidyl)-4-nitro-benzamide

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; HATU In ISOPROPYLAMIDE at 20℃; for 4h; | 78% |
| With N-ethyl-N,N-diisopropylamine; HATU In N,N-dimethyl-formamide at 18 - 25℃; for 18h; | 59% |
| With N-ethyl-N,N-diisopropylamine; HATU In N,N-dimethyl-formamide at 17 - 25℃; for 18h; | 59% |
| With N-ethyl-N,N-diisopropylamine; HATU In N,N-dimethyl-formamide at 18 - 25℃; for 18h; | 59% |
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In dichloromethane at 20 - 40℃; |
-
-
403-24-7
2-fluoro-4-nitrobenzoic acid

-
-
69411-67-2
2-fluoro-4-nitro-1-(trifluoromethyl)benzene

| Conditions | Yield |
|---|---|
| With sulfur tetrafluoride; hydrogen fluoride at 85℃; for 24h; Autoclave; Inert atmosphere; | 78% |
-
-
403-24-7
2-fluoro-4-nitrobenzoic acid

-
-
75-65-0
tert-butyl alcohol

-
-
157665-46-8
tert-butyl 2-fluoro-4-nitrobenzoate

| Conditions | Yield |
|---|---|
| With pyridine; p-toluenesulfonyl chloride at 0℃; for 19h; | 75% |
| With pyridine; p-toluenesulfonyl chloride at 0℃; for 19h; | 75% |
| With pyridine; p-toluenesulfonyl chloride 1.) 15 deg C, 5 min, 2.) RT, 2.5 h; Yield given. Multistep reaction; | |
| With pyridine; p-toluenesulfonyl chloride for 19h; | 524 mg |
-
-
870-46-2
t-butoxycarbonylhydrazine

-
-
403-24-7
2-fluoro-4-nitrobenzoic acid

-
-
590421-50-4
N'-(2-fluoro-4-nitrobenzoyl)hydrazinecarboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With 3-methyl-N-(3-methylbutyl)-1-butanamine; HATU In N,N-dimethyl-formamide at 20℃; | 57% |
| Stage #1: 2-fluoro-4-nitrobenzoic acid With N-ethyl-N,N-diisopropylamine; HATU In DMF (N,N-dimethyl-formamide) at 20℃; for 0.333333h; Stage #2: t-butoxycarbonylhydrazine In DMF (N,N-dimethyl-formamide) at 20℃; | 57% |
-
-
403-24-7
2-fluoro-4-nitrobenzoic acid

-
-
100-53-8
phenylmethanethiol

-
-
92163-95-6
2-(benzylsulfanyl)-4-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 65℃; for 35h; | 55.5% |
-
-
77668-69-0
(14S,16S,32R,33R,2R,4S,10E,12E,14R)-86-chloro-14-hydroxy-85,14-dimethoxy-33,2,7,10-tetramethyl-12,6-dioxo-7-aza-1(6,4)-oxazinana-3(2,3)-oxirana-8(1,3)-benzenacyclotetradecaphane-10,12-dien-4-yl methyl-L-alaninate

-
-
403-24-7
2-fluoro-4-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 20℃; for 72h; Inert atmosphere; | 35% |
2-Fluoro-4-nitrobenzoic acid Specification
The systematic name of this chemical is 2-fluoro-4-nitrobenzoic acid. With the CAS registry number 403-24-7, it is also named as 2-fluoro-4-nitrobenzenecarboxylic acid. And the molecular formula of this chemical is C7H4FNO4. It is a kind of white to light yellow crystal powder, and belongs to the following product categories: Aromatic Carboxylic Acids, Amides, Anilides, Anhydrides & Salts; Carboxylic Acids; Phenyls & Phenyl-Het; Benzoic acid; Miscellaneous.
The physical properties of 2-Fluoro-4-nitrobenzoic acid are as following: (1)ACD/LogP: 1.73; (2)# of Rule of 5 Violations: 0; (3)ACD/BCF (pH 5.5): 1; (4)ACD/BCF (pH 7.4): 1; (5)ACD/KOC (pH 5.5): 1; (6)ACD/KOC (pH 7.4): 1; (7)#H bond acceptors: 5; (8)#H bond donors: 1; (9)#Freely Rotating Bonds: 2; (10)Polar Surface Area: 83.12 Å2; (11)Index of Refraction: 1.588; (12)Molar Refractivity: 39.722 cm3; (13)Molar Volume: 118.014 cm3; (14)Polarizability: 15.747×10-24cm3; (15)Surface Tension: 62.642 dyne/cm; (16)Density: 1.569 g/cm3; (17)Flash Point: 167.007 °C; (18)Enthalpy of Vaporization: 63.037 kJ/mol; (19)Boiling Point: 352.534 °C at 760 mmHg; (20)Vapour Pressure: 0 mmHg at 25°C.
Preparation of 2-Fluoro-4-nitrobenzoic acid: This chemical can be prepared by 2-fluoro-4-nitro-toluene. The reaction will need reagent aq. KMnO4 and heating.
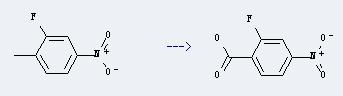
Uses of 2-Fluoro-4-nitrobenzoic acid: It can be used to produce 2-fluoro-4-nitro-benzoyl chloride. This reaction will need reagents (COCl)2 and DMF, and the solvent tetrahydrofuran. The reaction time is 3 hours with temperature of 0-20°C.

You should be cautious while dealing with this chemical. It irritates eyes, respiratory system and skin, and it is also harmful if swallowed. Therefore, you had better take the following instructions: Do not breathe dust; Wear suitable protective clothing, gloves and eye/face protection, and in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: c1cc(c(cc1[N+](=O)[O-])F)C(=O)O
(2)InChI: InChI=1/C7H4FNO4/c8-6-3-4(9(12)13)1-2-5(6)7(10)11/h1-3H,(H,10,11)
(3)InChIKey: MMWFMFZFCKADEL-UHFFFAOYAV
Related Products
- 2-Fluoro-4-nitrobenzoic acid
- 40326-95-2
- 40327-96-6
- 403-28-1
- 4032-86-4
- 403-29-2
- 403-31-6
- 40332-16-9
- 40332-17-0
- 40332-25-0
- 40332-58-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View